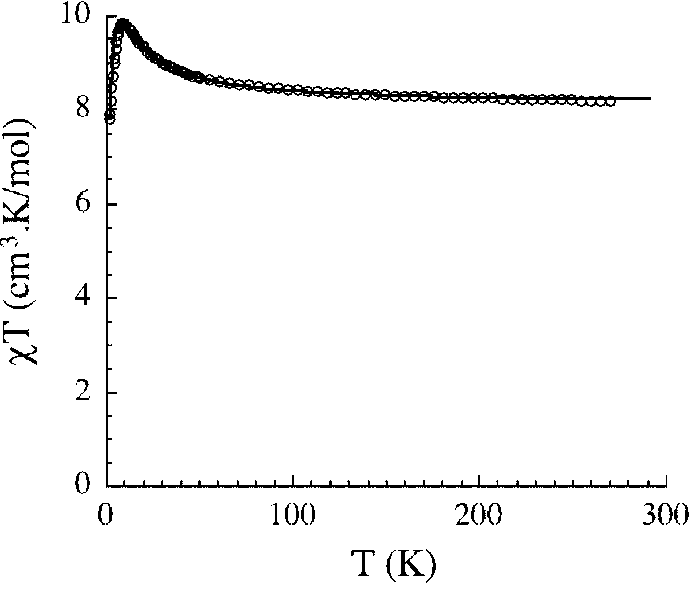
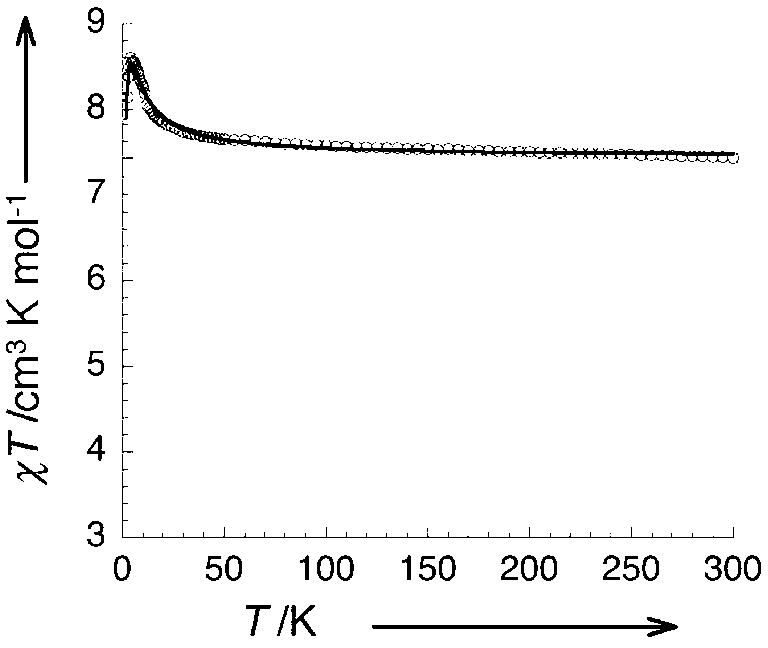
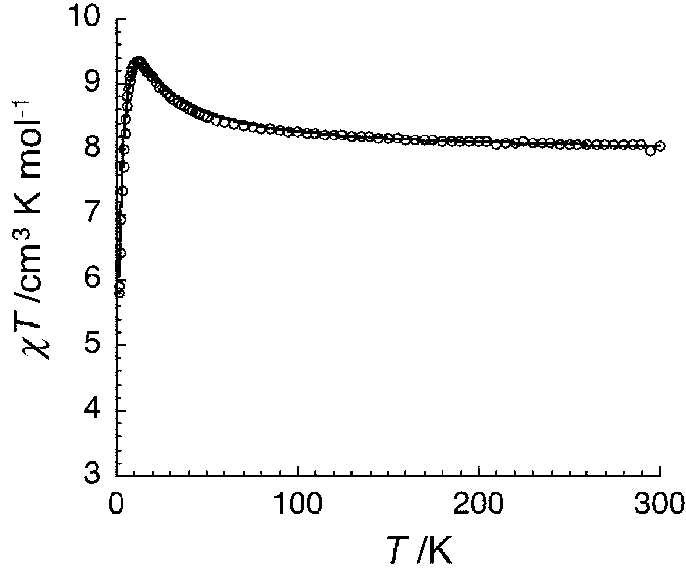
Structure-fine controllable Mn<2+> paramagnetic compound and its preparation method and use
A compound and synthesis method technology, applied in the field of Mn2 type paramagnetic compound and its preparation, can solve the problem of no paramagnetic compound structure and the like, and achieve the effects of low cost, abundant species and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The preparation method of the Mn2 type paramagnetic compound that can finely regulate the structure of the present invention comprises the following steps:
[0028] A, synthesize respectively the Salen ligand Mn-Salen type binuclear compound [Mn(salen)(H) modified by ethylenediamine, 1,2-propylenediamine, 1,3-propylenediamine 2 O)] 2 Na[CrMo 6 (OH) 6 o 18 ]·20H 2 O(1) 、 [Mn(salmen)(H 2 O)] 2 Na[CrMo 6 (OH) 6 o 18 ]·18H 2 O(2), [[Mn(salpn)(H 2 O)] 2 Na[CrMo 6 (OH) 6 o 18 ]·17H 2 O(3);
[0029] B. Dissolve three Mn-Salen type dinuclear compounds in C 2 h 5 OH-CH 3 OH-H 2 In the mixed solvent of O, obtain Mn-Salen solution;
[0030] C, polyanion compound is dissolved in water, obtain polyanion aqueous solution;
[0031] D. Drop the Mn-Salen solution into the polyanion aqueous solution, and stir at a constant speed at 40-50° C. for 4-10 hours to obtain the Mn2 type paramagnetic compound.
[0032]The synthetic route of the Schiff base ligand is as fol...
Embodiment 1
[0045] Dinuclear compound [Mn(salen)(H 2 O)] 2 Na[CrMo 6 (OH) 6 o 18 ]·20H 2 O(1) : 1.17g (1mmol) of Na 3 [CrMo 6 o 24 h 6 ]·8H 2 O was dissolved in 20ml of distilled water, and then dissolved with freshly prepared 0.88g (1mmol) [Mn(salen)(H 2 O)] 2 (ClO 4 ) 2 ·H 2 O 20ml of a mixed solution of methanol, ethanol and water was quickly added to the above solution, the mixed solution was sealed in an Erlenmeyer flask and stirred for 2 days, after filtration, the filtrate slowly evaporated at room temperature, dark brown flakes Crystalline 1 was formed within one week, and the yield was 35.8% based on Mo.
[0046] [Mn(salmen)(H 2 O)] 2 Na[CrMo 6 (OH) 6 o 18 ]·18H 2 O(2): 1.17g (1mmol) of Na 3 [CrMo 6 o 24 h 6 ]·8H 2 O was dissolved in 20ml of distilled water, and then dissolved with freshly prepared 0.88g (1mmol) [Mn(salmen)(H 2 O)] 2 (ClO 4 ) 2 ·H 2 A mixed solution of 20ml methanol, ethanol and water of O was quickly added to the above solution, a...
Embodiment 2
[0049] Dinuclear compound [Mn(salen)(H 2 O)] 2 Na[CrMo 6 (OH) 6o 18 ]·20H 2 O(1) : 1.17g (1mmol) of Na 3 [CrMo 6 o 24 h 6 ]·8H 2 O was dissolved in 20ml of distilled water, and then dissolved with freshly prepared 0.88g (1mmol) [Mn(salen)(H 2 O)] 2 (ClO 4 ) 2 ·H 2 O 20ml mixed solution of methanol, ethanol and water was quickly added to the above solution, the mixed solution was sealed in a conical flask and stirred for 2 days, after filtration, the filtrate slowly evaporated at room temperature, dark brown flakes The yield of crystal 1 was 37.8% based on Mo in two weeks.
[0050] [Mn(salmen)(H 2 O)] 2 Na[CrMo 6 (OH) 6 o 18 ]·18H 2 O(2): 1.17g (1mmol) of Na 3 [CrMo 6 o 24 h 6 ]·8H 2 O was dissolved in 20ml of distilled water, and then dissolved with freshly prepared 0.88g (1mmol) [Mn(salmen)(H 2 O)] 2 (ClO 4 ) 2 ·H 2 A mixed solution of 20ml methanol, ethanol and water of O was quickly added to the above solution, and the mixed solution was seale...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com