Ammonia synthesis catalyst and ammonia synthesis method
A technology for synthesis of catalysts and ammonia, applied in chemical instruments and methods, catalyst activation/preparation, physical/chemical process catalysts, etc., can solve the problems of high electronegativity of accelerators, and achieve the effect of simple manufacture and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085]
[0086] CaCO 3 and Al 2 o 3 The powders were mixed in such a way that the ratio of Ca to Al was 11:7, and heated in an alumina crucible at 1300°C for 6 hours. The obtained powder was inserted into a silica glass tube and placed in a 1×10 -4 Heated at 1100° C. for 15 hours in Pa vacuum. 3 g of the thus obtained powder and 0.18 g of metal Ca powder were inserted into a silica glass tube, heated at 700° C. for 15 hours to make the inside a metal Ca vapor atmosphere, and a conduction electron concentration of about 2×10 was obtained. 21 cm -3 C12A7:e - (denoted as C12A7e 21 ) powder.
[0087]
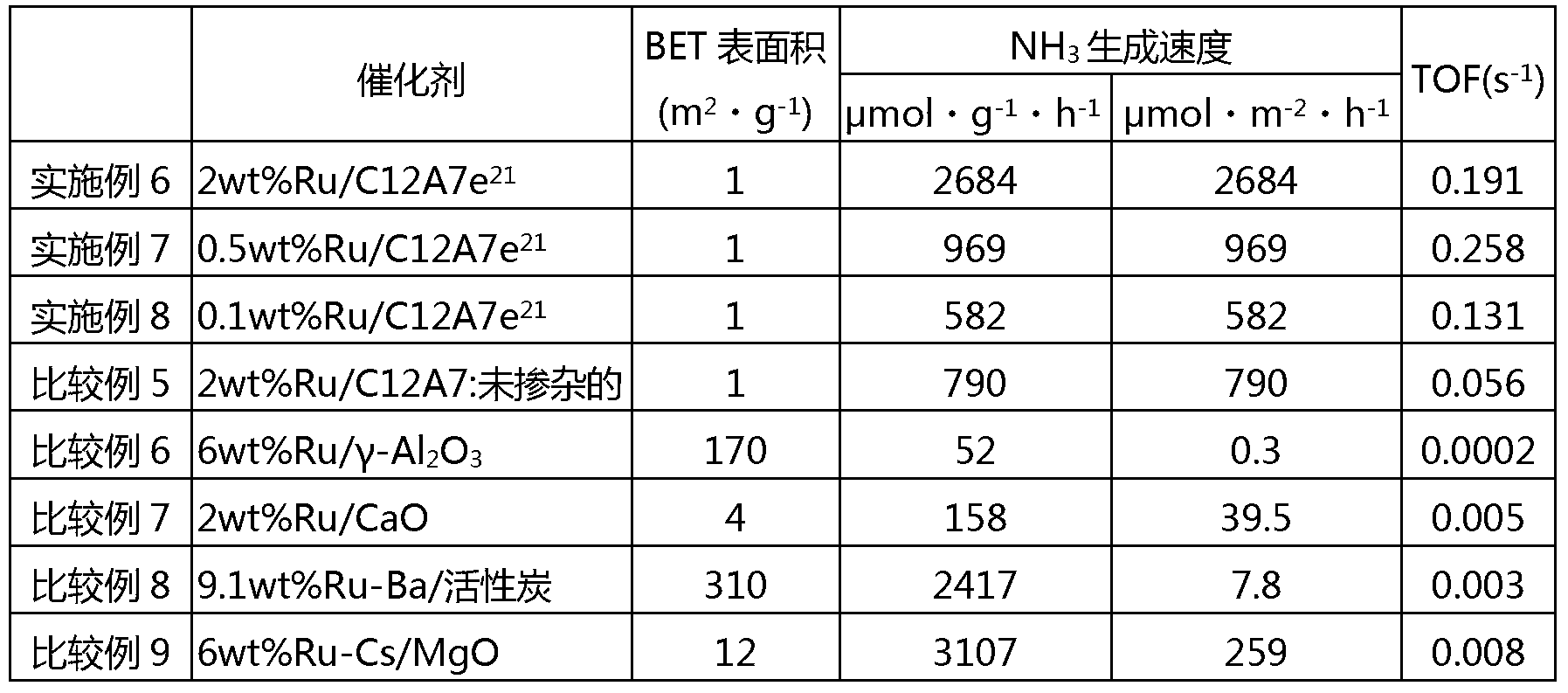
[0088] The obtained C12A7e 21 1g of powder and Ru dissolved in hexane solvent 3 (CO) 12 Mix and evaporate the solvent to dryness. At this time, according to the loading amount of Ru relative to C12A7e 21 The powder is 6wt% and the Ru in the solvent is adjusted 3 (CO) 12 amount. The catalyst precursor was formed by heating the obtained powder at 100°C under vacuum ...
Embodiment 2
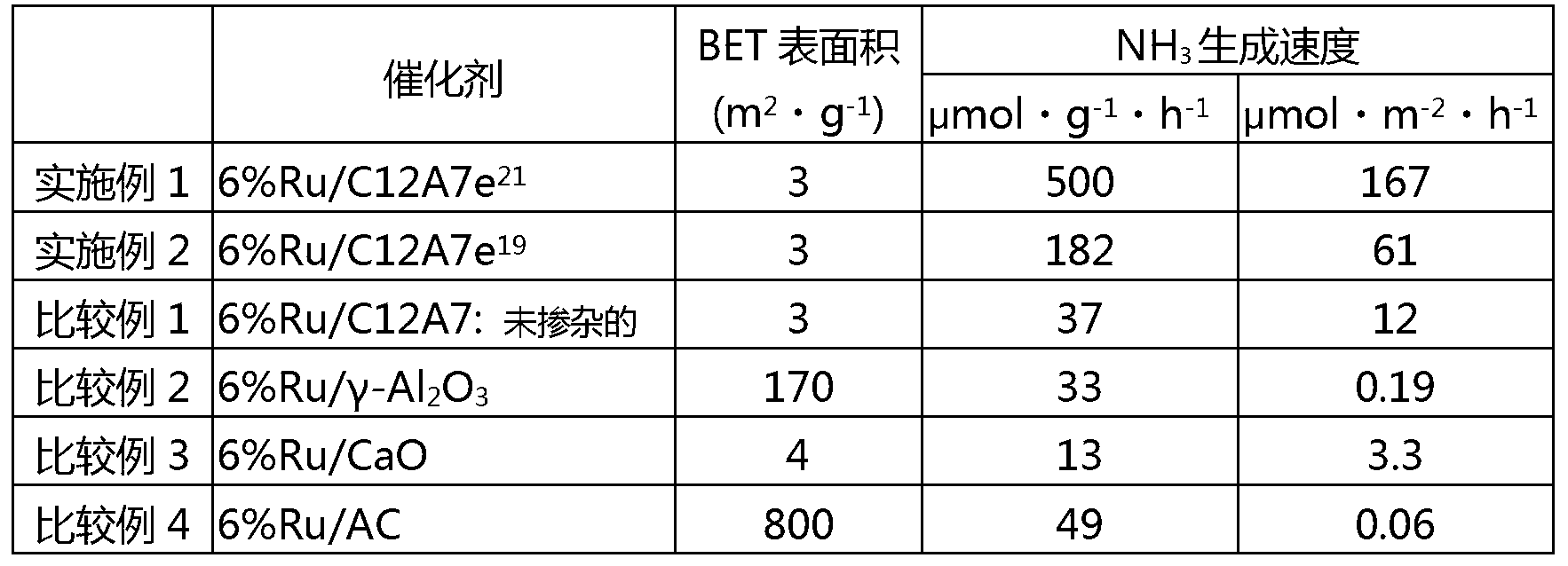
[0092] used a 1×10 19 cm -3 The chemical equivalent of electrons composed of C12A7 (C12A7e 19 ), except that, the ammonia synthesis reaction was carried out under the same conditions as in Example 1. The ammonia production rate is shown in Table 1.
Embodiment 3
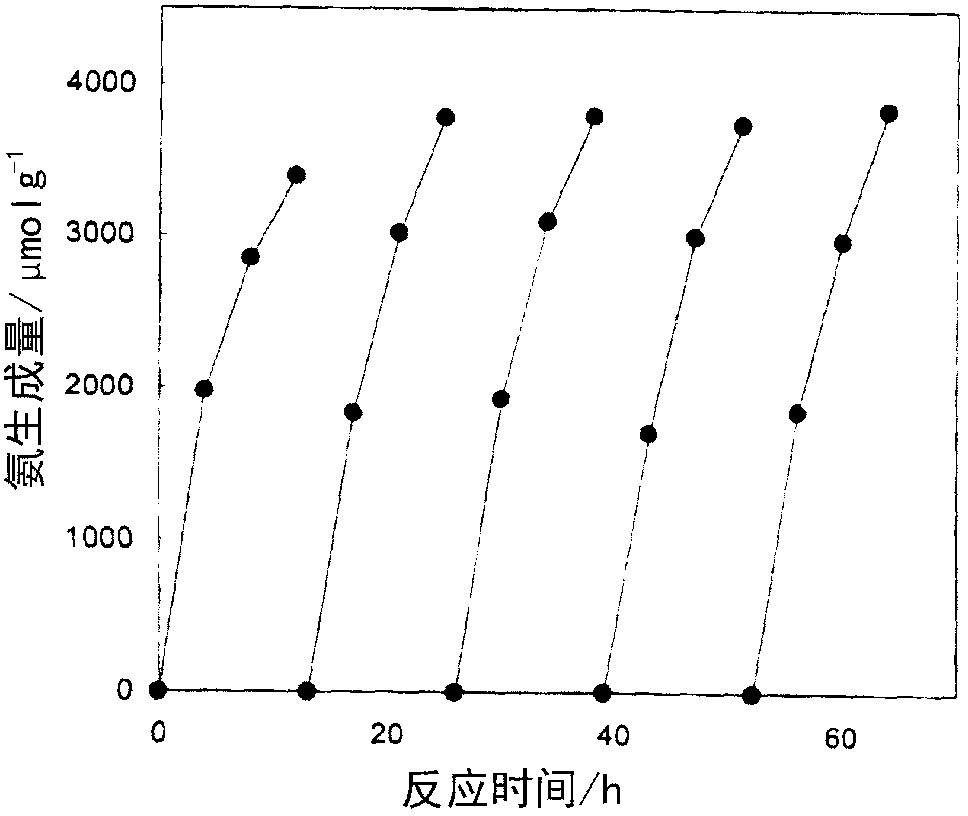
[0105] Using the same conditions as in Example 1, after carrying out the synthesis reaction for more than ten hours, the reaction system was vacuum exhausted, and then the N 2 6.7kPa and H 2 20.0kPa was introduced into the reaction system, and the synthesis reaction was carried out again at 400°C for more than ten hours. The stability of the catalyst was evaluated by further repeating this operation three times. figure 1 Given in Ru / C12A7e 21 The result obtained by repeating the synthesis reaction as a catalyst. From the left: 1st, 2nd, 3rd, 4th, and 5th. It is clear that even if the synthesis reaction is repeated five times, no decrease in the catalyst activity is observed, and the synthesis reaction proceeds under catalysis. This shows that the present catalyst does not show deterioration during the synthesis reaction and is stable even when used for a long period of time.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com