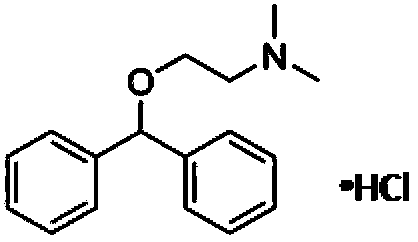
Application of diphenhydramine hydrochloride in preparation of medicines for treating or preventing influenza viruses
A technology of diphenhydramine hydrochloride and influenza virus, applied in the field of medicine, can solve the problem of no diphenhydramine hydrochloride against influenza virus and the like, and achieve the effect of broad-spectrum antiviral activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: Evaluation of anti-influenza virus activity of diphenhydramine hydrochloride
[0033] 1. Experimental materials and methods
[0034] 1.1 Cells, viruses and drugs
[0035] MDCK cells were purchased from ATCC, virus A / PR8 / 34H1N1 was amplified from chicken embryo culture, and diphenhydramine hydrochloride was purchased from Sigma;
[0036] 1.2 Experimental Instruments
[0037] Multifunctional detector PerkinElmer, inverted microscope Costar
[0038] 2. experimental method:
[0039] 2.1 Cell culture:
[0040] 37°C, 5% (volume ratio, the same below) CO 2 cultured in a humidified incubator. A DMEM medium containing 10% (volume ratio, the same below) FBS, 100 U / mL of penicillin and streptomycin was used. Cells were subcultured after reaching 90% confluence, and the subculture ratio was 1 / 3–1 / 4.
[0041] 2.2 Virus culture:
[0042] Take 9-11 day-old SPF chicken embryos, check them with an egg tester before inoculating the virus, and mark them at a posit...
Embodiment 2
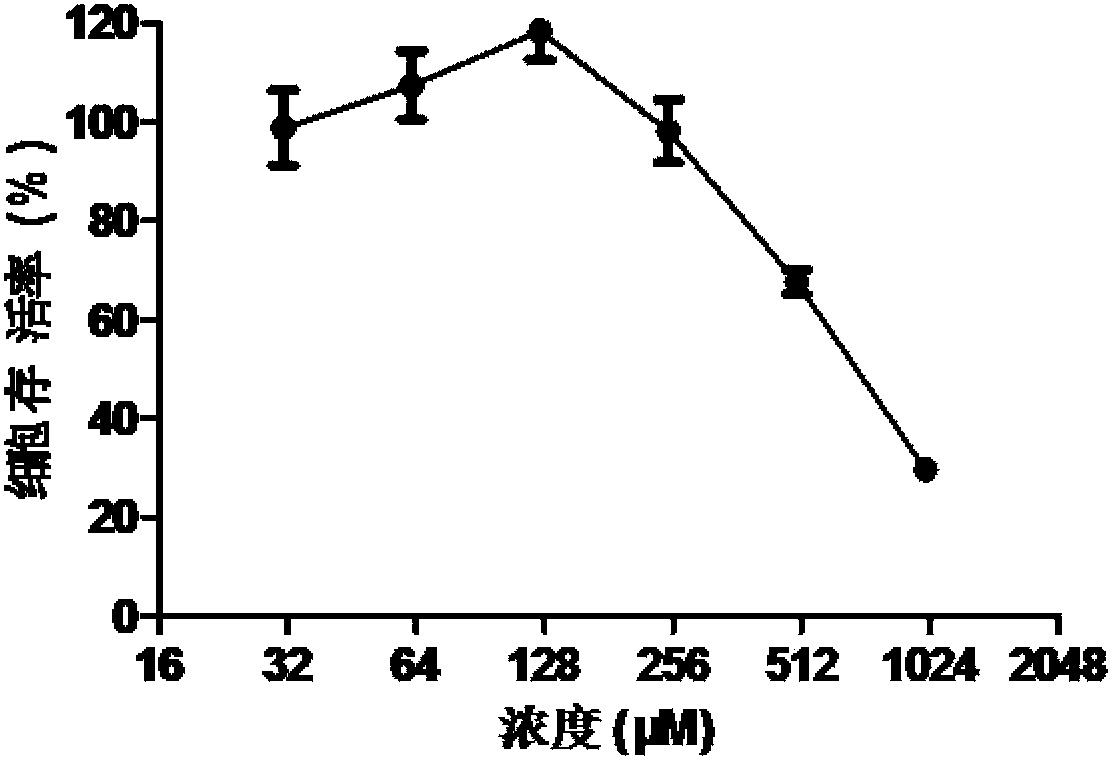
[0057] Embodiment 2: the inhibitory effect of diphenhydramine hydrochloride on different stages of virus replication cycle
[0058] 1. cells by 5*10 4 Inoculate in 24-well cell culture plates, and wait for the cells to adhere to the wall and form a monolayer for later use. The life cycle of influenza virus is about 6 hours, so we gave 50μM drug culture at virus infection -2~0h, 0~2h, 2~4h, 4~6h respectively, fixed the cells at 6h after infection, and performed indirect immunofluorescence according to the following steps The expression levels of viral proteins after drug treatment were detected.
[0059] 2. Discard the supernatant of the culture medium, fix the cells with fixative solution at room temperature for 15-20min; wash 3 times with PBS, 5min each time; monoclonal antibody) and incubate at room temperature for 1 h; wash with PBS 3 times, 5 min each time; add fluorescently labeled secondary antibody diluted in binding solution and DAPI staining solution and incubate ...
Embodiment 3
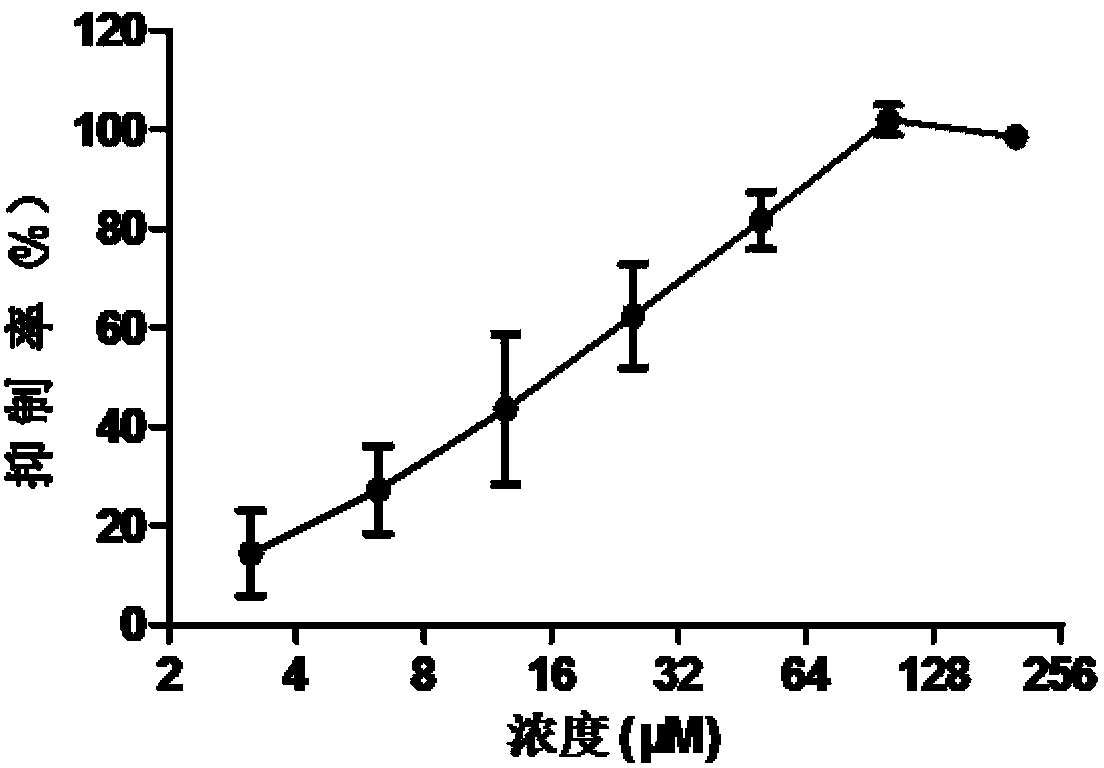
[0062] Example 3: Study on broad-spectrum anti-influenza virus activity of diphenhydramine hydrochloride
[0063] In this experiment, the antiviral activity of diphenhydramine hydrochloride on influenza A virus subtype H1N1 strain, influenza A virus H3N2 strain and influenza B virus strain was detected.
[0064] 1. The virus strains used in the experiment include: A / human / Hubei / 1 / 2009 (H1N1), A / human / Hubei / 3 / 2005 (H3N2), Influenza B virus
[0065] 2. Divide MDCK cells into 2*10 4 Cells / well were seeded in 96-well cell culture plates, cultured in a cell culture incubator at 37°C for 14-18 hours, and the cells were grown into a single layer before use. Discard the culture medium in the well plate, wash it twice with PBS, add 100TCID50 / well virus solution to infect the cells, and add drugs of various concentration gradients at the same time (starting at 200μM, serially dilute 6 gradients by 2 times, each gradient 2 A total of 200 μl of maintenance medium (DMEM+0.3%BSA+2.5ug / L...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com