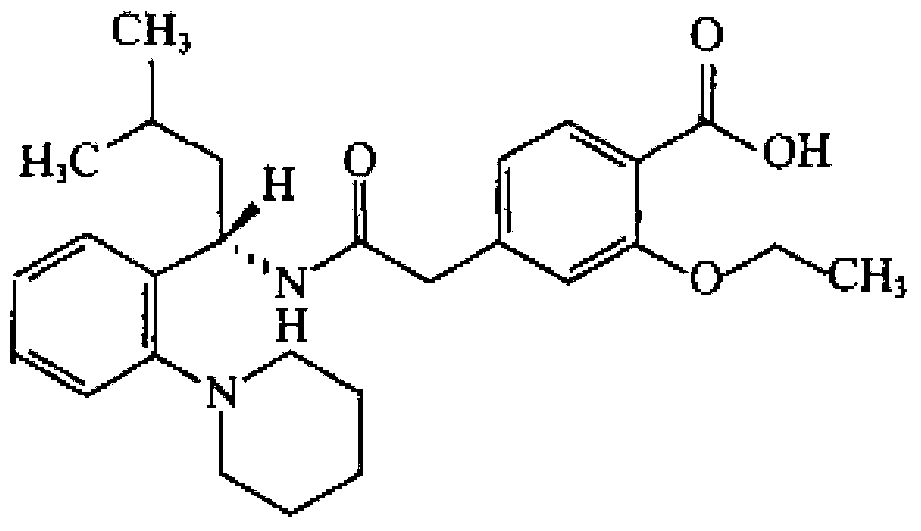
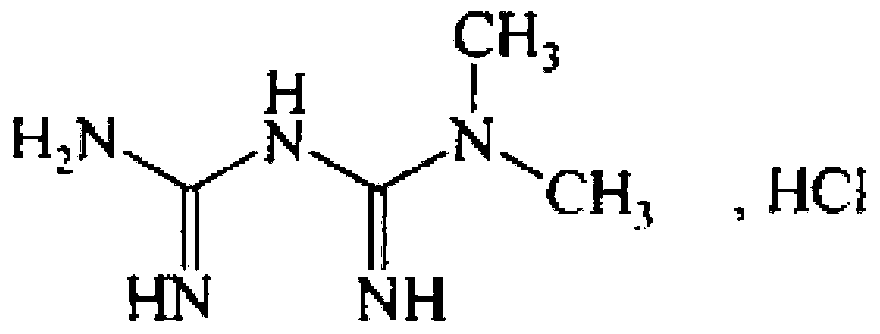
Repaglinide/metformin composition
A technology of metformin hydrochloride and its composition, which is applied in the field of medicine and can solve the problems of high tablet hardness, inconsistent onset time, and high treatment costs for patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
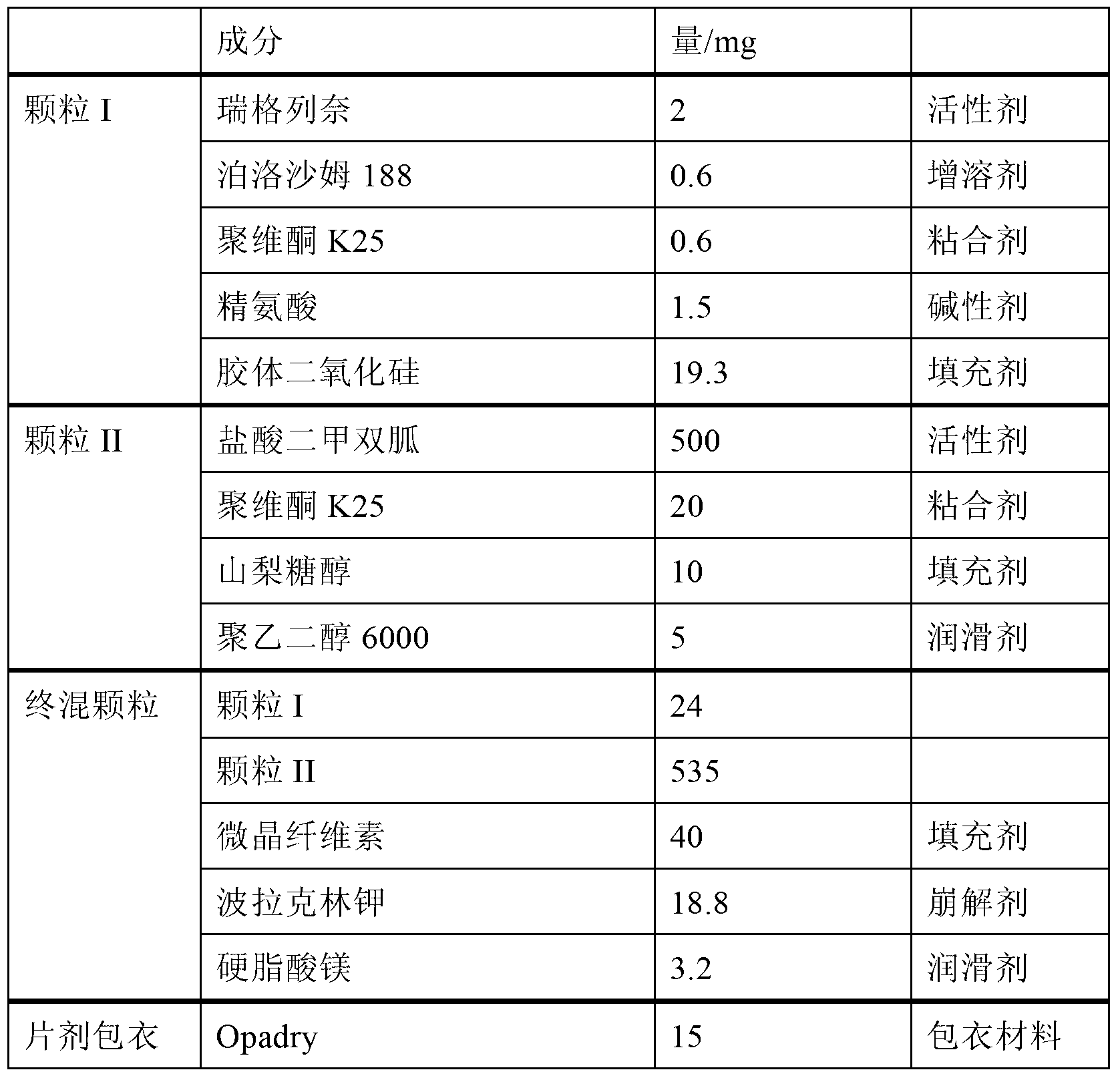
[0079] Embodiment 1: preparation pharmaceutical composition
[0080] formula:
[0081]
[0082] In the above table, the amount of arginine is 1.95 mole times that of repaglinide (both are expressed in mole times when calculating the relative amounts of the two below).
[0083] Preparation method:
[0084] (a) Grinding each material to a pharmaceutically acceptable particle size (particle size less than 80 mesh);
[0085] (b) Preparation of granule I: Repaglinide, alkaline agent, solubilizer, and binder are dissolved in an appropriate amount of water to make a 2 / 4 saturated solution, and the solution is sprayed (retrofitted syringe at the outlet, solution can be ejected from it when extruded outwards) into the filler, fully mixed evenly, dried to a particle with moisture lower than 2%, and then the dried particle is ground into a fine powder with a particle size of less than 80 mesh to obtain particle I (this process The repaglinide yield of medium granule I is 96.7%); ...
Embodiment 2
[0090] Supplementary Example 2: Referring to the formula and method of the above-mentioned Example 1, the difference is that the amount of colloidal silicon dioxide is adjusted, which are 0, 1, 2.5, 5, 7.5, 12.5, 15, 20, 30, and 50 times by weight, the plain tablets obtained are respectively recorded as Ex121, Ex122, Ex123, Ex124, Ex125, Ex126, Ex127, Ex128, Ex129 and Ex130, and are sealed and packed in aluminum-plastic composite film bags respectively.
Embodiment 3
[0091] Supplementary Example 3: Referring to the formula and method of the above-mentioned Example 1, the difference is that arginine is replaced by meglumine, and the amounts are 0.5, 1.0, 2.0, 5.0, 10.0 mole times of repaglinide respectively , The plain sheets obtained are recorded as Ex131, Ex132, Ex133, Ex134, Ex135 respectively, and are sealed and packed in aluminum-plastic composite film bags respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com