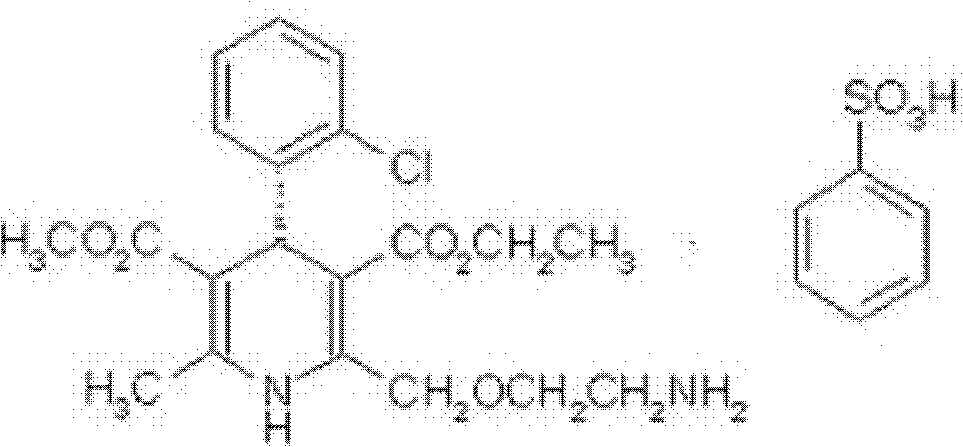
Brand new medicinal composition containing levamlodipine and losartan potassium and preparation method thereof
A kind of technology of levamlodipine and levamlodipine besylate, applied in the new medicinal composition containing levamlodipine and losartan potassium and the field of preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] [embodiment 1] preparation of levamlodipine besylate crystal
[0081] 1) dissolving levamlodipine besylate in a mixed solvent of dichloromethane and ethanol to obtain a dichloromethane / ethanol solution of levamlodipine besylate;
[0082] 2) Add n-heptane dropwise to the dichloromethane / ethanol solution of levamlodipine besylate obtained in step 1) under an ultrasonic field until crystallization occurs;
[0083] 3) Turn off the ultrasonic field, let stand, filter, wash the filter cake with dichloromethane and ethanol respectively, and dry to obtain the levamlodipine besylate crystal.
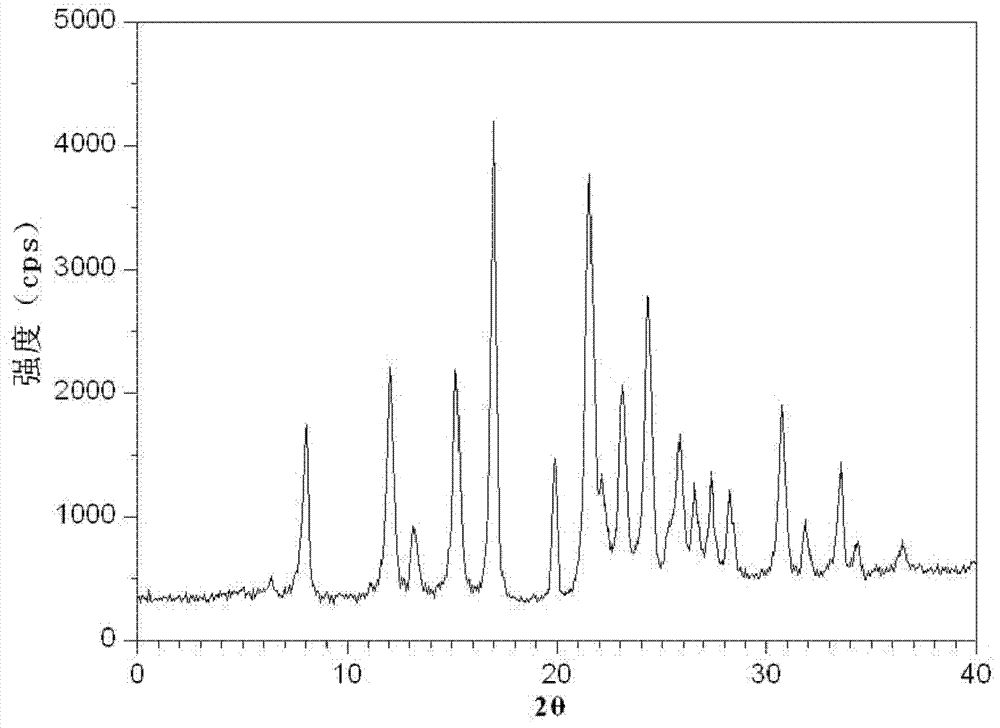
[0084] Gained levamlodipine besylate crystals use Cu-Kα rays to measure characteristic peaks in the X-ray powder diffraction pattern obtained at 2θ of 8.0°, 12.1°, 15.4°, 17.0°, 19.8°, 21.6°, 23.0° , 24.3°, 25.7°, 27.4°, 30.7° and 33.5° display, such as figure 1 shown.
[0085] Below is embodiment 2-9, and preparation method is with embodiment 1, and its concrete process parameter is sh...
Embodiment 1
[0098] [Preparation Example 1] Levoamlodipine Besylate / Losartan Potassium Tablets
[0099] The prescription is the same as the pharmaceutical composition Example 1, the prescription is to make 1000 tablets, and its preparation method is to further carry out the following steps 4) and step 5) to the standby mixed powder obtained in step 3) to obtain tablets:
[0100] 4) Sampling and testing the mixed powder, calculating the tablet weight according to the content determination results, and performing direct powder compression of the obtained pharmaceutical composition powder;
[0101] 5) Prepare the coating solution, adjust the inlet air temperature to 60°C, preheat the plain tablet temperature to 50°C, adjust the compressed air pressure to 0.35MPa, make the sprayed coating solution into a fine mist, and perform film coating. Coating time was 1.5 hours, and the tablet weight gain was 0.16%. Stop coating, adjust the air inlet temperature to 60° C., and continue drying for 10 minu...
Embodiment 2
[0102] [Preparation Example 2] Levoamlodipine Besylate / Losartan Potassium Tablets
[0103] The prescription is the same as the pharmaceutical composition example 2, and the prescription is to make 1000 tablets, and its preparation method is to further carry out the following steps 4) and step 5) to the standby mixed powder obtained in step 3) to obtain tablets:
[0104] 4) Sampling and testing the mixed powder, calculating the tablet weight according to the content determination results, and performing direct powder compression of the obtained pharmaceutical composition powder;
[0105] 5) Prepare the coating solution, adjust the inlet air temperature to 60°C, preheat the plain tablet temperature to 45°C, adjust the compressed air pressure to 0.25MPa, make the sprayed coating solution into a fine mist, and perform film coating. The coating time was 2 hours, and the tablet weight gain was 0.08%. Stop coating, adjust the air inlet temperature to 60°C, and continue drying for 10 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com