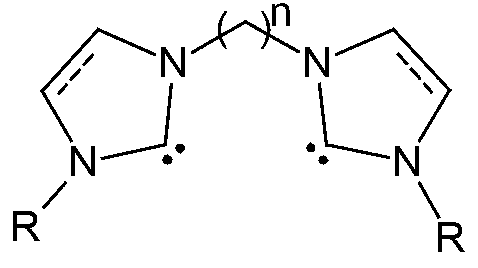
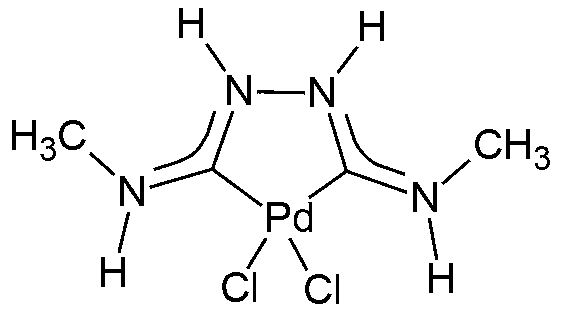
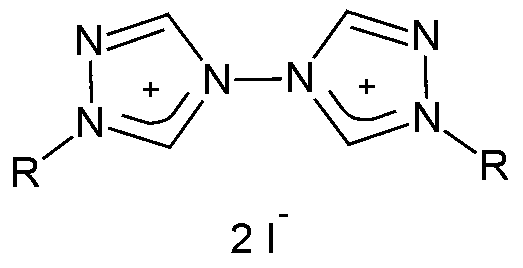
Metal complexes
A compound and metal atom technology, applied in the direction of gold organic compounds, osmium organic compounds, copper organic compounds, etc., can solve the problems that salts cannot deprotonate double carbene, limit, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0224] Example 1: 3,6-bis(chloromethyl)pyridazine
[0225]
[0226] 3,6-Dimethylpyridazine (6.60 g, 61.0 mmol) in anhydrous CHCl 3 (400ml) was heated to boiling under a protective gas atmosphere. Trichloroisocyanuric acid (12.3 g, 52.9 mmol) was then added in small portions over the course of one hour. In each case, the mixture foamed briefly and a brown suspension formed which was heated to reflux for an additional 2.5 hours. After cooling to room temperature, the light brown solid was separated through a filter (D4) and the filtrate was washed twice with 150 ml each of 0.2M aqueous NaOH and twice each with 200 ml water. Na 2 SO 4 After drying, the solvent was removed in vacuo to leave a brown oil, to which a small amount of pentane was added and the mixture was stored overnight at -30°C. The brown crystals formed were filtered and extracted with 50 ml anhydrous diethyl ether. The ether insoluble brown solid was discarded and the ether solution was evaporated to dryn...
Embodiment 2
[0234] Example 2: 3,6-bis(n-propylaminomethyl)pyridazine
[0235]
[0236] Under protective gas, n-propylamine (1.85ml, 1.33g, 22.6mmol) was added to 3,6-bis(chloromethyl)pyridazine (200mg, 1.13mmol) from Example 1 in anhydrous acetonitrile (5ml) in the solution. The brown solution was stirred overnight at room temperature, then evaporated to dryness. The residue was extracted twice with 5 ml each of a mixture of diethyl ether and acetonitrile (1:1 ) and once with diethyl ether (5 ml). The solution was evaporated in vacuo and the oily residue was recrystallized from diethyl ether at -30°C. The product was obtained as a yellow-brown solid (174 mg, 69%).
[0237] 1 H-NMR (CD 3 CN,400.13MHz):
[0238] δ=7.56(s,2H,4 / 5-H),3.99(s,4H,NCH 2 ),2.54(t, 3 J HH =7.1Hz,4H,NCH 2 -Pr),1.91(s,br,2H,NH),1.48(sext, 3 J HH =7.3Hz,4H,CH 2 -Pr),0.90(t, 3 J HH =7.4Hz,6H,CH 3 -Pr).
[0239] 13 C{ 1 H}-NMR (CD 3 CN,100.61MHz):
[0240] δ=162.7(C3 / 6), 127.5(C4 / 5), 54.3(NCH 2 ),...
Embodiment 9
[0244]Example 9: 3,6-bis(n-propylcarboxamidomethyl)pyridazine
[0245]
[0246] Under argon atmosphere, 3,6-bis(n-propylaminomethyl)pyridazine (1.60 g, 7.18 mmol) from Example 2 was added to formic acid (purity 99+%, 10 ml) and acetic anhydride (1.5 ml ) in the mixture. A small amount of gas evolved and a yellow-brown solution formed which was stirred at room temperature for 1.5 hours. Then 5 ml of water were added and the solution was evaporated to dryness in vacuo. The oily brown residue was extracted five times with 10 ml of diethyl ether each time. Using Na 2 SO 4 After drying, the ether solution was evaporated to dryness in vacuo to give the product as a light yellow oil in the form of its three isomers A, B and C, which was used without further purification (1.75 g, 88%) .
[0247] 1 H-NMR (CD 3 CN,400.13MHz):
[0248] (signals of isomers A, B and C assigned as best as possible)
[0249] δ=8.34, 8.33, (both s, 3H, CHO, A and B), 8.21, 8.20 (both s, 3H, CHO, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com