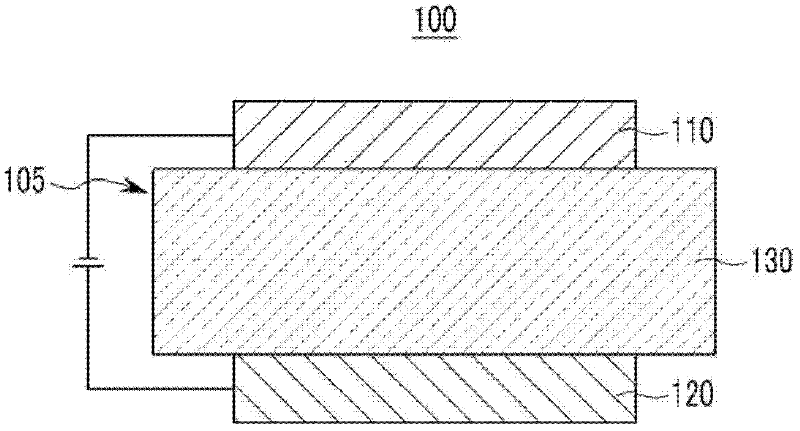
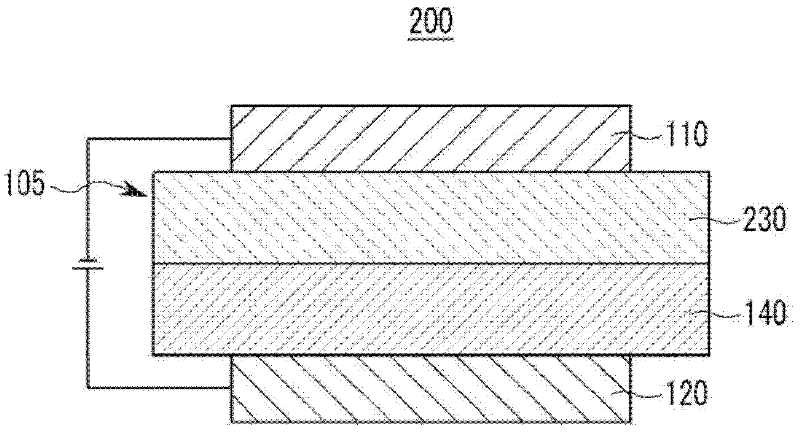
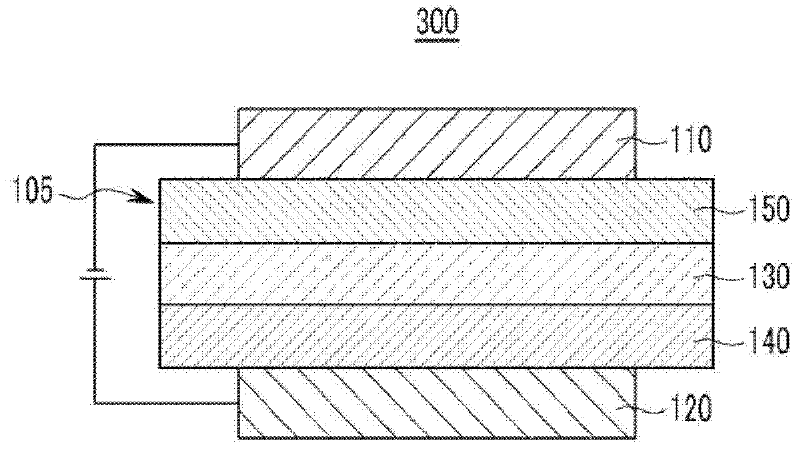
Compound for an organic photoelectric device, and organic photoelectric device comprising same
A technology of organic optoelectronic devices and compounds, which can be used in organic dyes, lighting devices, photovoltaic power generation, etc., can solve problems such as unsatisfactory development, and achieve the effects of excellent electrochemical and thermal stability and excellent lifespan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0123] Embodiment 1: synthesis is represented by the compound of chemical formula 12
[0124] As an example of a compound for an organic photoelectric device in the present invention, a compound represented by Chemical Formula 12 was synthesized according to Reaction Scheme 1.
[0125]
[0126] Step 1: Synthesis of compound (A)
[0127] Mix 5 g (15.5 mmol) of 3-bromo-6-phenylcarbazole, 6.3 g (17.1 mmol) of 3-phenyl-6-carbazole boric acid pinacol ester in a 250 mL round bottom flask using a stirrer under nitrogen atmosphere And 100mL tetrahydrofuran mixed with 2M potassium carbonate aqueous solution. The mixture was heated to reflux for 12 hours under nitrogen flow. After the reaction was completed, hexane was added to the reactant. The solid generated therein was then filtered and dissolved in a mixed solution prepared by mixing toluene and tetrahydrofuran at a volume ratio of 50:50, to which activated carbon and anhydrous magnesium sulfate were added. Then stir the m...
Embodiment 2
[0132] Embodiment 2: synthesis is represented by the compound of chemical formula 26
[0133] As one example of the compound for the organic photoelectric device in the present invention, the compound represented by Chemical Formula 26 was synthesized according to Reaction Scheme 2.
[0134]
[0135] Step 1: Synthesis of compound (B)
[0136] Mix 2g (4.13mmol) of intermediate product represented by general formula A, 2.3g (6.2mmol) of 3-bromo-N-phenylcarbazole, 0.2g (2.1mmol) of copper chloride, 1.7mL in a 100mL round bottom flask g (12.4 mmol) of potassium carbonate, 0.37 g (2.1 mmol) of 1,10-phenanthroline and 80 mL of dimethyl sulfoxide, and heated at 180° C. for 24 hours under nitrogen flow. Thereafter, the organic solvent was removed by distillation under reduced pressure, and 2 g of compound B was obtained by column chromatography (67% yield).
[0137] Step 2: Synthesis of Chemical Formula 26
[0138] Add 2g (2.76mmol) intermediate product represented by compou...
Embodiment 3
[0141] Embodiment 3: synthesis is represented by the compound of chemical formula 10
[0142] As one example of the compound for the organic photoelectric device in the present invention, the compound represented by Chemical Formula 10 was synthesized according to Reaction Scheme 3.
[0143]
[0144] In the 100mL round bottom flask, add 2g (2.76mmol) intermediate product represented by compound B, 1.3g (4.1mmol) N-(4-bromophenyl) diphenylamine, 0.14g (1.4mmol) copper chloride, 1.14 g (8.3 mmol) of potassium carbonate, 0.25 g (1.4 mmol) of 1,10-phenanthroline and 80 mL of dimethylsulfoxide and heated at 180° C. for 24 hours under nitrogen flow. Then the organic solvent was removed by distillation under reduced pressure, and 2 g of compound 8 was obtained by column chromatography (yield 75%).
[0145] The compound represented by Chemical Formula 10 was subjected to elemental analysis. The result is as follows.
[0146] Calculate C 72 h 48 N 4 : C, 89.23; H, 4.99; N, 5.7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com