High-efficiency and clean tungsten smelting method
A smelting method and clean technology, applied in the field of tungsten smelting, can solve the problem of high cost and achieve the effect of reducing costs and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
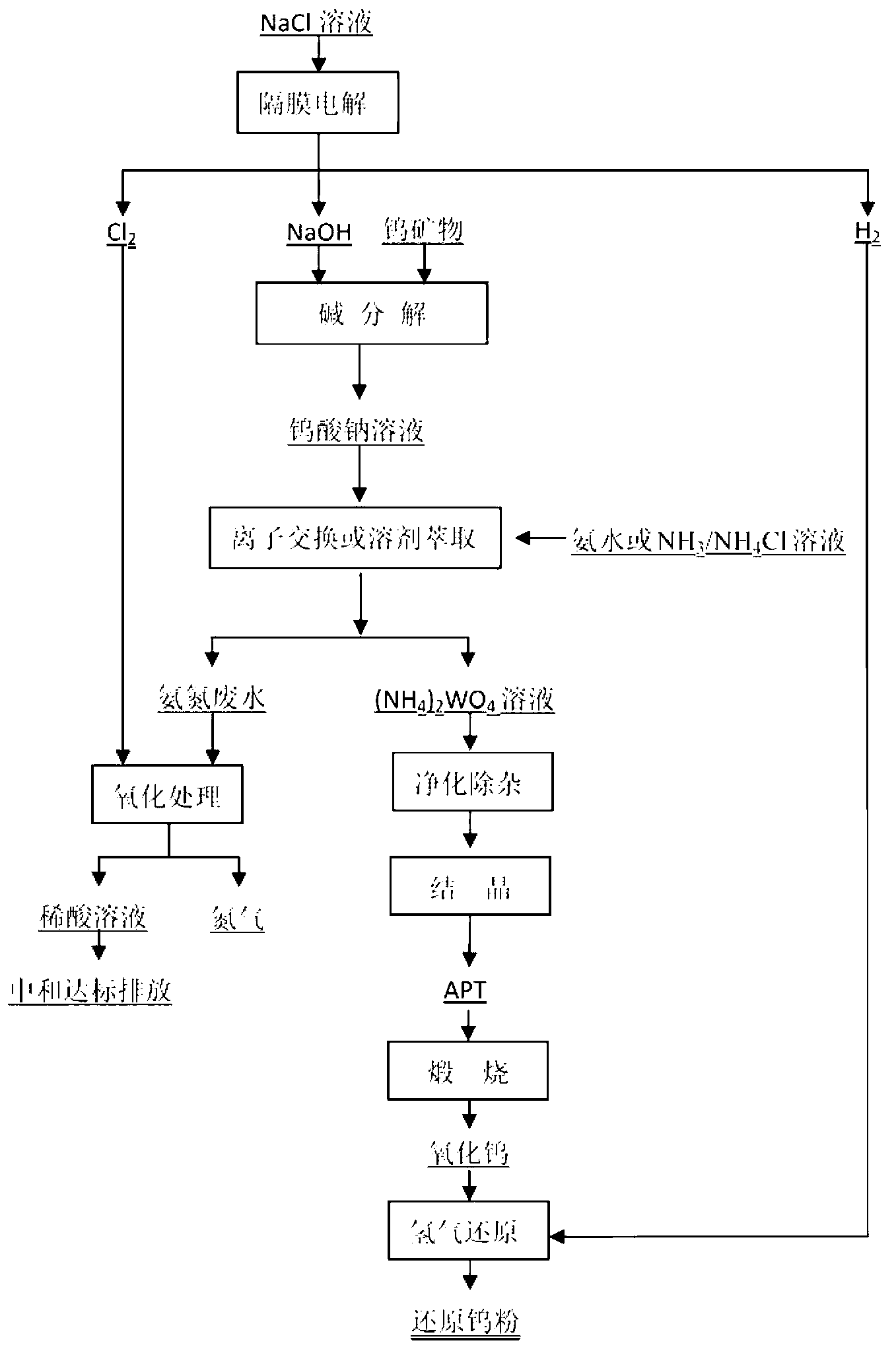
[0056] The NaCl solution with a mass concentration of 26% was electrolyzed to obtain a NaOH solution with a concentration of 30%, which was reacted with wolframite with a grade of 60% according to 1.5 times the theoretical consumption, the liquid-solid ratio was 0.9:1, and the reaction temperature was controlled at 160 ℃, the reaction time is 2.0 hours, the leaching rate reaches 99.2%, and 180g / L Na 2 WO 4 solution. Dilute the solution to 25g / L, use 201×7 resin for adsorption, after washing completely, then use 5mol / LNH 4 Cl+2mol / LNH 3 h 2 O is desorbed to obtain a concentration of 256g / L ammonium tungstate solution. After ion exchange, the liquid and resin washing liquid are added by Cl 2 Feed Cl in a molar ratio of 1.02 to ammonia in the solution 2 Oxidation, converting ammonia nitrogen into N 2 The concentration of ammonia nitrogen in the discharge liquid is 0.3mg / L. The ammonium tungstate solution is evaporated and crystallized, and the crystallization rate is cont...
Embodiment 2
[0058] The NaCl solution with a mass concentration of 26% was electrolyzed to obtain a NaOH solution with a concentration of 28%, which was reacted with scheelite with a grade of 58% according to the theoretical consumption of 3.5 times, the solid-liquid ratio was 1:1, and the reaction temperature was controlled at 200°C, the leaching rate reaches 98.5%, and 220g / L Na 2 WO 4 solution. Use HCl to adjust the pH value of the solution to 4.5, use D301 resin for adsorption, and then use 25% ammonia water for desorption to obtain a concentration of 256g / L ammonium tungstate solution. After ion exchange, the liquid and resin washing liquid are added by Cl 2 Feed Cl with a molar ratio of 1.03 to ammonia in the solution 2 Oxidation, converting ammonia nitrogen into N 2 remove. After the reaction is completed, the ion-exchanged liquid is NaCl with a concentration of 112g / L, which is evaporated to saturation and then returned for electrolysis; the ammonia nitrogen concentration in t...
Embodiment 3
[0060] The NaCl solution with a mass concentration of 26% is electrolyzed to obtain a NaOH solution with a concentration of 28%. According to the theoretical consumption of 4.0 times, it reacts with scheelite with a grade of 61%. The solid-liquid ratio is 1.1:1, and the reaction temperature is controlled at 180°C, the leaching rate reaches 98.7%, and 215g / L Na 2 WO 4 solution. solution using H 2 SO 4 Adjust the pH value to 4.2, use D314 resin for adsorption, and then use 25% ammonia water for desorption to obtain a concentration of 256g / L ammonium tungstate solution. Resin use H 2 SO 4 After transformation, it is used to adsorb tungsten. The liquid after ion exchange, the resin washing liquid and the liquid after transformation are added according to Cl by the break point chlorination method 2 Feed Cl with a molar ratio of 1.1 to ammonia in the solution 2 Oxidation, converting ammonia nitrogen into N 2 remove. After the reaction is completed, the concentration of amm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com