Stable fructose composition preparation and preparation method thereof
A technology of composition and fructose, which is applied in the direction of drug combination, medical preparations of non-active ingredients, non-active ingredients of polymer compounds, etc., can solve the problems of large pH value changes, failure to meet quality requirements, poor stability, etc., to achieve The preparation method is simple, the drug availability is improved, and the safe dosage is large
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
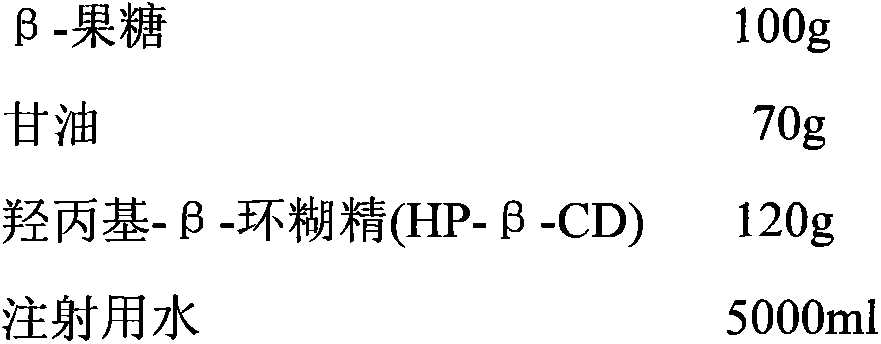
[0044]
[0045] Preparation Process:
[0046] Add sterile β-fructose to the reaction kettle at room temperature and suspend it in water for injection; add sterile glycerin to adjust the pH value and dissolve it completely to obtain an aqueous solution of fructose / glycerin; After adding, adjust the pH value to 6.0 to dissolve all β-fructose; add hydroxypropyl-β-cyclodextrin (HP-β-CD) to the reaction kettle, stir and clathrate for 1.0 hour; add water for injection to dilute to the required quality concentration, then filtered through a 0.45 μm microporous membrane, and finally filtered through a 0.22 μm microporous membrane, subpackaged, and freeze-dried to obtain the fructose freeze-dried powder for injection.
Embodiment 2
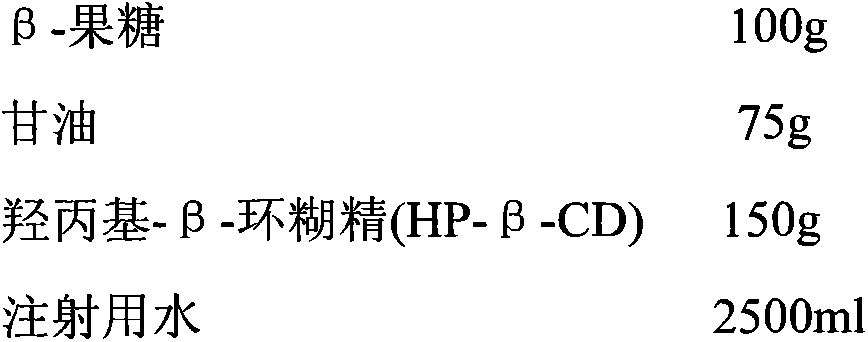
[0048]
[0049] Preparation Process:
[0050] Add sterile β-fructose to the reaction kettle at room temperature and suspend it in water for injection; add sterile glycerin to adjust the pH value and dissolve it completely to obtain an aqueous solution of fructose / glycerin; After adding, adjust the pH value to 6.5 to completely dissolve the sterile β-fructose; add hydroxypropyl-β-cyclodextrin (HP-β-CD) to the reaction kettle, stir and clathrate for 1.5 hours; add water for injection to dilute to the The mass concentration is required, then filtered through a 0.45 μm microporous membrane, and finally filtered through a 0.22 μm microporous membrane, and then packaged to obtain fructose injection.
Embodiment 3
[0052]
[0053] Preparation Process:
[0054] Add sterile β-fructose to the reaction kettle at room temperature and suspend it in water for injection; add sterile glycerin to adjust the pH value and dissolve it completely to obtain an aqueous solution of fructose / glycerin; After adding, adjust the pH value to 7.0 to completely dissolve the sterile β-fructose; add hydroxypropyl-β-cyclodextrin (HP-β-CD) to the reaction kettle, stir and clathrate for 2.0 hours; add water for injection to dilute to the The mass concentration is required, then filtered through a 0.45 μm microporous membrane, and finally filtered through a 0.22 μm microporous membrane, subpackaged, and freeze-dried to obtain the fructose freeze-dried powder for injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com