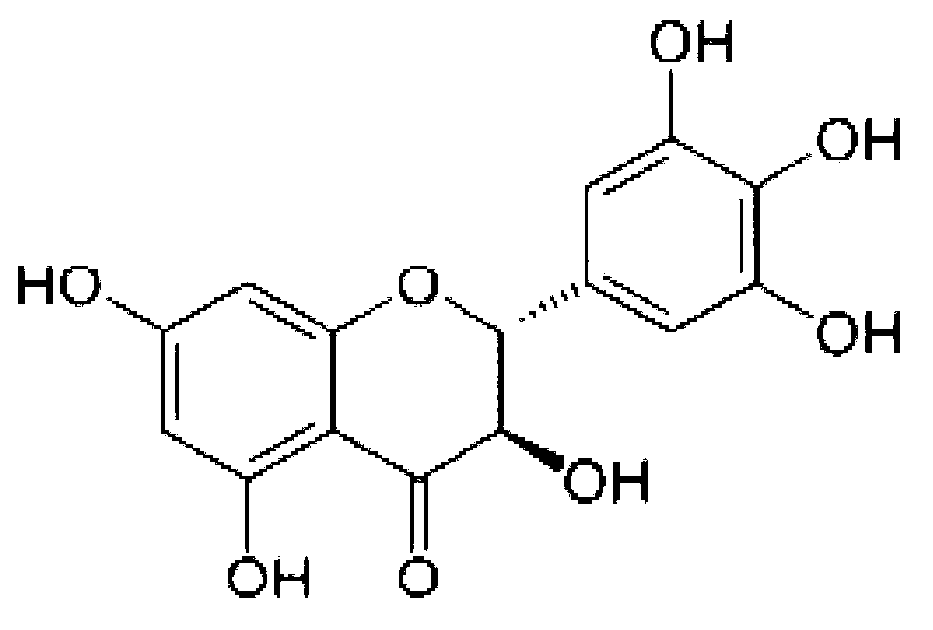
Application of dihydromyricetin (DHM) in preparation of anti-hepatoma medicines
A technology of dihydromyricetin and anti-liver cancer, applied in the field of biomedicine, can solve the problem of no anti-liver cancer drug of dihydromyricetin, and achieve the effects of inducing apoptosis of liver cancer cells, inhibiting proliferation, and having strong anti-cancer properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1D
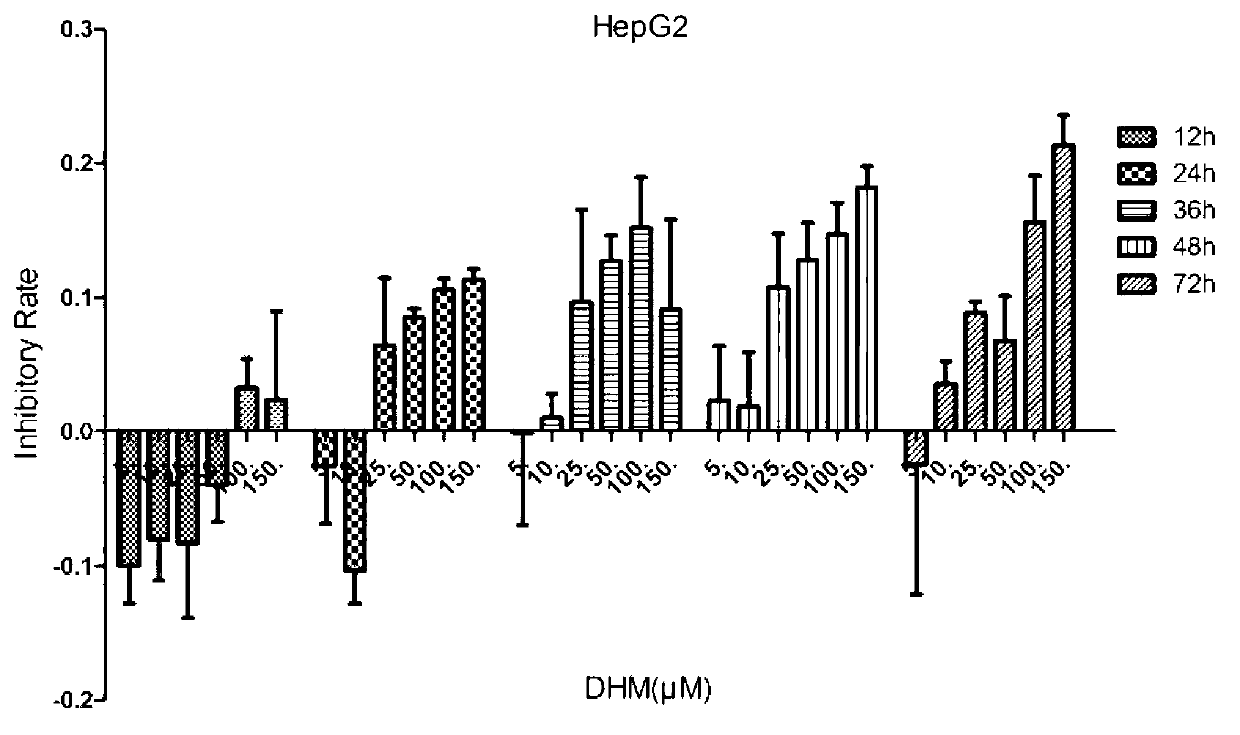
[0097] Example 1 Effect of DHM on proliferation of liver cancer cell lines
[0098] 1. In vitro culture of liver cancer cell lines
[0099] 1. Cell source
[0100] Human liver cancer cell lines HepG2, Huh7, SK-HEP-1 were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences; MHcc97L, MHcc97H, QSG7701, QGY7703 were purchased from Shanghai Baili Biotechnology Co., Ltd.; QGY7701 was purchased from Shanghai Maternal and Child Health Hospital; human normal liver The cell line L-02 was purchased from Science Cell; the mouse liver cancer cell line Hepal-6 was donated by Shanghai Jiaotong University.
[0101] 2. Cell Culture and Subculture
[0102] HepG2, QSG7701, L-02 and Hepal-6 used 1640 medium (Gibco); Huh7, SK-HEP-1, MHcc97L, MHcc97H, QGY7701 and QGY7703 used DMEM medium (Gibco). 10% fetal bovine serum (fetal bovine serum, FBS, Gibco) was added to the medium during cell culture. The above cell lines were all stored at 37°C, 5% CO 2 Culture in an incubator...
Embodiment 2D
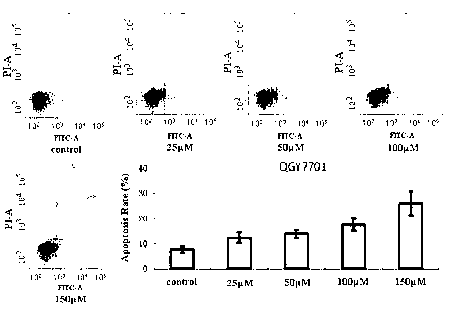
[0111] Example 2 Induction of DHM on apoptosis of liver cancer cells
[0112] 1. Cell culture and passage
[0113] In this experiment, the human liver cancer cell line QGY7701 was purchased from Shanghai Maternal and Child Health Hospital. The culture medium was DMEM culture solution supplemented with 10% FBS. 2 Culture in an incubator, and when the cells grow to 80-90% of the bottom of the culture dish, they are digested with 0.25% trypsin-EDTA and passaged.
[0114] 2. The effect of DHM on the apoptosis of liver cancer cells
[0115] Main test materials: DHM, dissolved in dimethyl sulfoxide (DMSO), prepared as a 50mM concentrated solution, stored at -20°C, diluted to 25, 50, 100 and 150μM when used, ready to use; FITC Annexin Ⅴ Apoptosis Detection Kit Ⅰ (BD Pharmingen); Accutase-Enzyme Cell Detachment Medium (eBioscience); PBS; 60mm petri dish, special sample tube for flow cytometry.
[0116] Test method: Digest the cells into a cell suspension, spread them on a 60mm cult...
Embodiment 3
[0118] Example 3 Western Blotting Detection of Cell Apoptosis-Related Factor Expression
[0119] 1. Cell culture and passage
[0120] The human liver cancer cell line HepG2 was purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences; QSG7701 and QGY7703 were purchased from Shanghai Baili Biotechnology Co., Ltd.; the mouse liver cancer cell line Hepal-6 was donated from Shanghai Jiao Tong University. HepG2, QSG7701 and Hepal-6 used 1640 medium (Gibco), and QGY7703 used DMEM medium (Gibco). 10% fetal bovine serum (fetal bovine serum, FBS, Gibco) was added to the medium during cell culture. The above cell lines were all stored at 37°C, 5% CO 2 Culture in an incubator, and when the cells grow to 80-90% of the bottom of the culture dish, they are digested with 0.25% trypsin-EDTA and passaged.
[0121] 2. Expression detection of apoptosis-related factors
[0122] Main test materials: DHM, dissolved in dimethyl sulfoxide (DMSO), prepared into a 50mM concentrated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com