Application of benzamide derivative to preparing antitumor drug
An anti-tumor drug, benzamide technology, applied in the chemical industry, can solve problems such as adverse reactions of anti-tumor drugs, and achieve the effects of not easy drug resistance, obvious effect, and obvious tumor inhibition effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Virtual screening of CypJ small molecule inhibitors
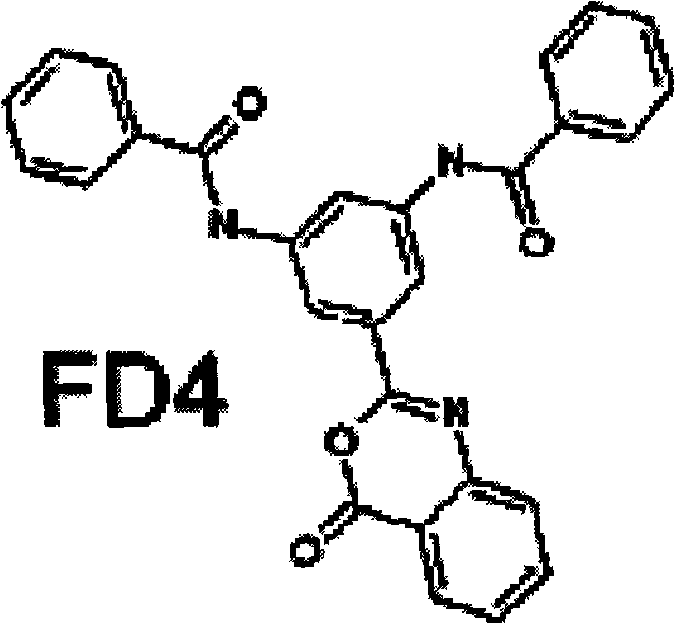
[0028] In the PDB protein structure database, the X-ray diffraction crystal structure of human CYPA protein was retrieved (PDB code: 1CWA). This structure is a complex crystal structure of CYPA and its natural inhibitor cyclosporin A (CsA). From this structure, the active site of CYPA was determined, and several key amino acid sites in the active site that can be inhibited by CsA were determined. Based on the highly homologous sequence of CYPJ and CYPA, we cooperated with the Shanghai Institute of Materia Medica, Chinese Academy of Sciences to establish a 3D structure model, which was also confirmed during subsequent protein crystallization and structural analysis (CYPJ PDB code: 1XYH). For the active site of CypJ, several small molecule databases were screened. The small molecule databases used for screening mainly include SPECS and CNPD. Finally, FD4 of the present invention was selected. The whole calcu...
Embodiment 2
[0029] Example 2 Using BIAcore molecular interaction instrument to verify virtual screening results
[0030] The BIAcore molecular interaction instrument is based on surface plasmon resonance technology to track the interaction between biomolecules, without any markers, so the authenticity of the experimental results is guaranteed to the greatest extent. In the experiment, the target biomolecule (CypJ protein) is fixed on the surface of the sensor chip, and then the small molecule compound is dissolved in the solvent and flows over the surface of the chip. The monitor can track the changes in the whole process of binding and dissociating the molecules in the detection solution to the target biomolecules on the chip surface in real time. According to the binding data of BIAcore, the N-[3-(benzamide)-5-(4-oxo-4H-3,1-benzoxazin-2-yl)phenyl]benzamide of the present invention and CypJ binding equilibrium-dissociation constant KD(M): 1.17×10 -5 .
Embodiment 3
[0031] Example 3 Using enzyme activity experiments to prove the ability of small molecule compounds to inhibit CypJ enzyme activity
[0032] There are many methods to determine CyP activity, but the α-chymotrypsin-coupled enzymic assay is the most commonly used. The principle is that oligopeptide substrates containing proline, such as N-succinyl-Ala-Ala-Pro-Phe (N-Succinyl-Ala-Ala-Pro-Phep-nitroanilide) in the solution CyP can catalyze the cis-trans isomerization of the substrate, that is, catalyze the proline from cis to trans; when it is in the trans structure, the C-terminal p-nitroanilide is α- The chymotrypsin cleaves to release the pigment group p-nitroaniline. The PPIase activity of CyP can be obtained by continuously measuring the change in absorbance at 390nm.
[0033] We used the reaction without adding small molecule inhibitors as a control reaction to determine the inhibitory rate of the small molecule ligand on the enzyme activity at different concentrations, and then...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com