K99-987P-F41 recombinant protein and application thereof
A recombinant protein, F41 technology, applied in the field of animal molecular biology and genetic engineering, can solve the problems of loss of important antigens, incomplete immunity, and high production cost of inactivated vaccines, achieve elimination of side reactions, high specificity, and overcome the problem of whole bacteria. Effects of inactivated vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
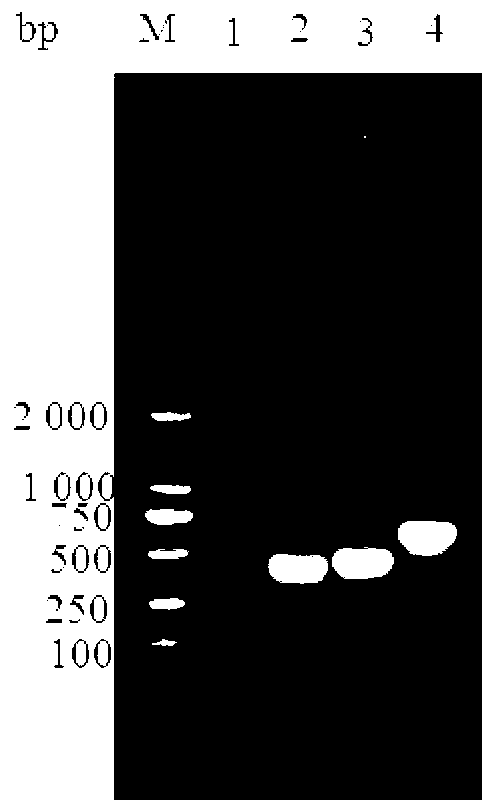
[0045] Primers were designed according to the complete sequences of enterotoxigenic Escherichia coli adhesin K99, 987P, and F41 genes published in GenBank (accession numbers M35282.1, M35257.1, and M21788.1, respectively). The genome of Bacillus C83919 was used as a template, and target fragments were amplified by PCR respectively; among them, Escherichia coli C83912, Escherichia coli C83916, and Escherichia coli C83919 contained adhesin K99, adhesin 987P, and adhesin F41 respectively, and the sequences of the primers used were as follows:
[0046] P1: K99(F): CGG GGTACC ATGACTATTAACTTCAATGGCAAA (KpnI) (SEQ ID No. 3);
[0047] P2: K99(R): CGC GGATCC TGAAGTAGTAAATACGCCAGC (BamHI) (SEQ ID No. 4);
[0048] P3: 987P(F): CGC GGATCC GGTGGTGGTGGTTCTGCTGAAAACAACACCAGCCAG (BamHI) (SEQ ID No. 5);
[0049] P4: 987P (R): C GAGCTC ACTAGCAGTGACAGTACCGGC (SacI) (SEQ ID No. 6);
[0050] P5: F41(F): C GAGCTC GGCGGTGGCGGCAGCGTATCTGGTTCAGTGATGGCT (SacI) (SEQ ID No. 7);
[0051] P6: F...
Embodiment 2
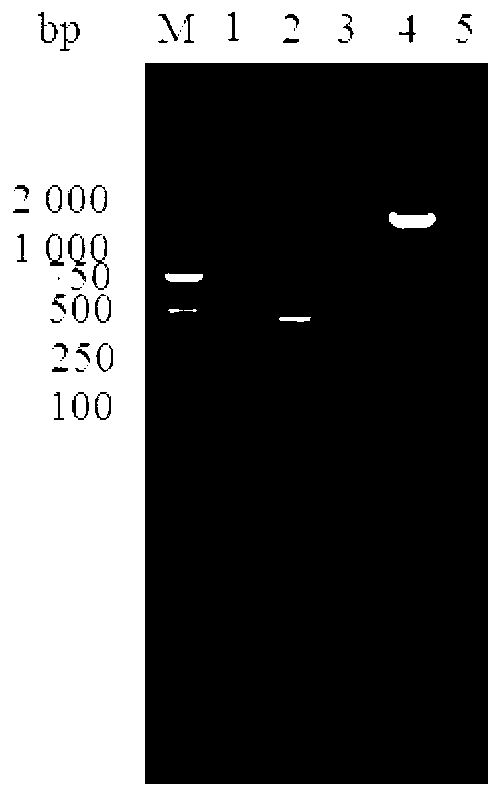
[0108] Using the same method as in Example 1, 987P was amplified using P3 / P4 and using the Escherichia coli C83916 genome as a template. The gel was recovered, connected to pMD-18T, and transformed into DH5α competent cells. After identification by PCR and double enzyme digestion, the positive plasmids were named pMD-18T-987P-DH5α respectively. pMD-18T-987P-DH5α and pET30a-K99 were digested with BamHI and SacI, the target fragments were recovered by gel cutting, and then ligated to construct pET30a-K99-987P, which was identified by PCR and double digestion. F41 was amplified using P5 / P6 and using the Escherichia coli C83919 genome as a template. The gel was recovered, connected to pMD-18T, and transformed into DH5α competent cells. After identification by PCR and double enzyme digestion, the positive plasmids were named pMD-18T-F41-DH5α respectively. pMD-18T-F41-DH5α and pET30a-K99-987P were digested with SacI and NotI, the target fragments were recovered by gel cutting, and ...
Embodiment 3
[0110] This example illustrates the induced expression of recombinant proteins.
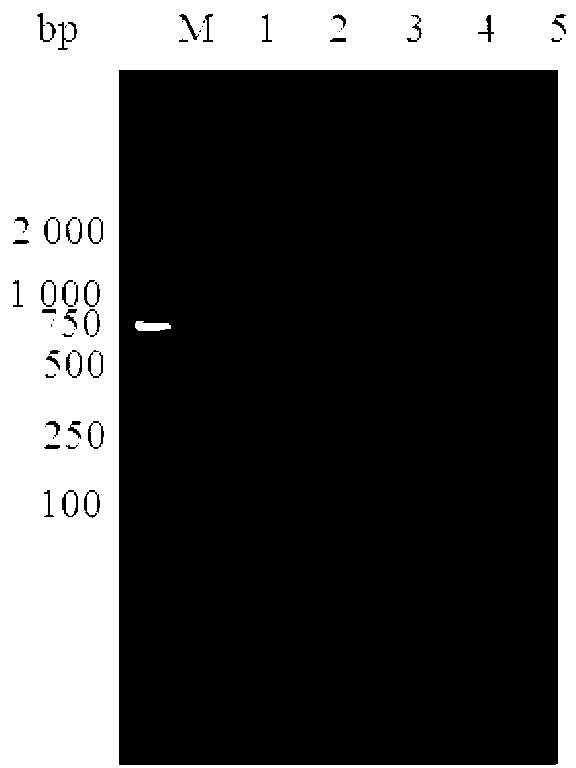
[0111] The positive recombinant expression plasmid pET30a-K99-987P-F41-BL21(DE3) was transformed into Escherichia coli BL21(DE3), and a single colony was picked and cultured with shaking in LB liquid medium containing Kana at 37°C. When the OD600 value reached 0.6, IPTG was added to a final concentration of 0.5 mmol / L for induction. At the same time, the expression vector control group was set, and the bacterial liquid was taken 5 hours after induction of expression, centrifuged at 12000r / min for 1min, and the precipitate was preserved. PBS resuspended bacteria pellet, after ultrasonic lysis, centrifuged at 10000r / min for 20min, collected supernatant and precipitate for SDS-PAGE electrophoresis, the results are as follows Figure 4 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com