Novel method for removing protein impurities in groups A, C meningococcal capsular polysaccharide
A technology of meningococcal and capsular polysaccharides, which is applied to protein impurities in the capsular polysaccharides of group C meningococci and removes the A field, which can solve the problems of high cost, great harm, and incapability of large-scale production, and achieve low production costs , simple operation, suitable for large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
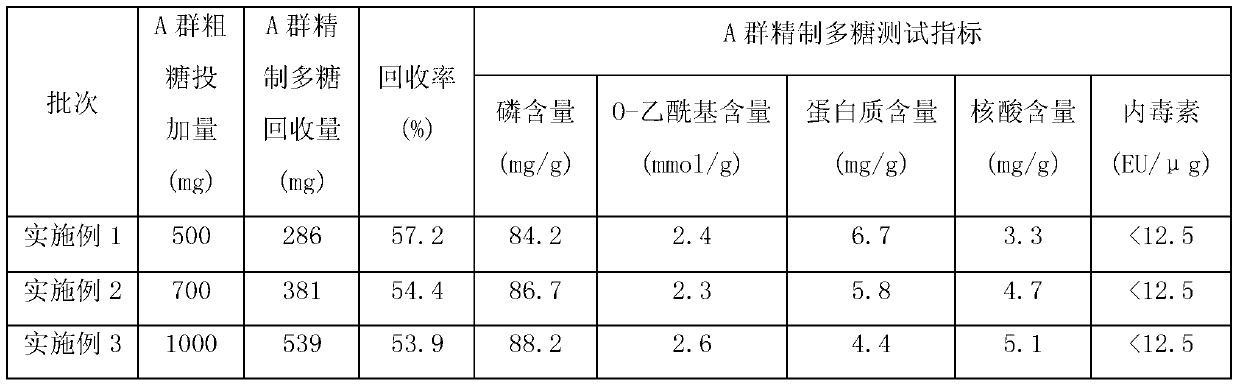
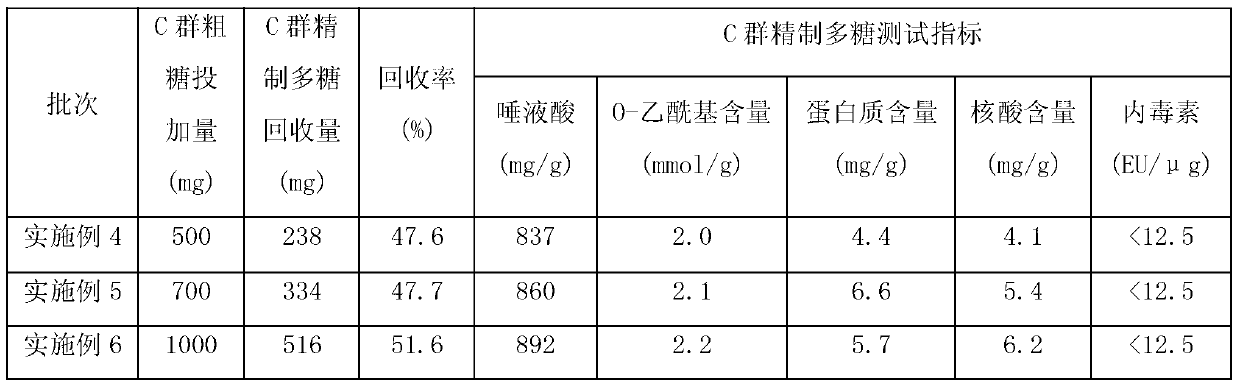
[0013] Embodiment 1: the new method of removing protein impurity in the capsular polysaccharide of group A meningococcus, it comprises the following steps:
[0014] S1. Dissolve 500 mg of the crude Group A epidemic cerebrospinal meningitis capsular polysaccharide with water for injection, dilute it into a polysaccharide solution of 5 mg / ml, add 2%, sodium deoxycholate solution of pH8.0, and the volume ratio is 0.5: 1. Mix well and stir at 2°C for 0.5h;
[0015] S2. Adjust the pH to 2 with 1mol / L hydrochloric acid, stir at 2°C for 3min, and then centrifuge at 8000rpm for 1.5h;
[0016] S3. Take the supernatant, adjust the pH to 7 with 1mol / L sodium hydroxide, and repeat steps S1 and S2 once;
[0017] S4. Take the supernatant, adjust the pH to 7 with 1mol / L sodium hydroxide, dilute 5 times with water for injection, ultrafilter to the original volume through a 250kd ultrafiltration membrane, repeat dilution, ultrafiltration concentration twice, and the last ultrafiltration conce...
Embodiment 2
[0018] Embodiment 2: the new method of removing protein impurity in the capsular polysaccharide of group A meningococci, it comprises the following steps:
[0019] S1. Dissolve 700 mg of crude Group A epidemic cerebrospinal meningitis capsular polysaccharide with water for injection, dilute it into a polysaccharide solution of 10 mg / ml, add 2%, sodium deoxycholate solution of pH8.0, and the volume ratio is 1.5: 1. Mix well and stir at 6°C for 1.5h;
[0020] S2. Adjust the pH to 3 with 1mol / L hydrochloric acid, stir at 6°C for 7min, and then centrifuge at 8000rpm for 1.5-2.5h;
[0021] S3. Take the supernatant, adjust the pH to 7 with 1mol / L sodium hydroxide, and repeat steps S1 and S2 once;
[0022] S4. Take the supernatant, adjust the pH to 7 with 1mol / L sodium hydroxide, dilute 15 times with water for injection, ultrafilter to the original volume through a 350kd ultrafiltration membrane, repeat dilution, ultrafiltration concentration 5 times, and the last ultrafiltration co...
Embodiment 3
[0023] Embodiment 3: the new method of removing protein impurity in the capsular polysaccharide of group A meningococci, it comprises the following steps:
[0024] S1. Dissolve 1000 mg of crude Group A meningococcal meningitis capsular polysaccharide with water for injection, dilute it into a polysaccharide solution of 8 mg / ml, add 2%, sodium deoxycholate solution of pH8.0, and the volume ratio is 1: 1. Mix well and stir at 4°C for 1 hour;
[0025] S2. Adjust the pH to 2 with 1mol / L hydrochloric acid, stir at 4°C for 5min, and then centrifuge at 8000rpm for 2h;
[0026] S3. Take the supernatant, adjust the pH to 7 with 1mol / L sodium hydroxide, and repeat steps S1 and S2 once;
[0027] S4. Take the supernatant, adjust the pH to 7 with 1mol / L sodium hydroxide, dilute 10 times with water for injection, ultrafilter to the original volume through a 300kd ultrafiltration membrane, repeat dilution, ultrafiltration concentration 3 times, and the last ultrafiltration concentration to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com