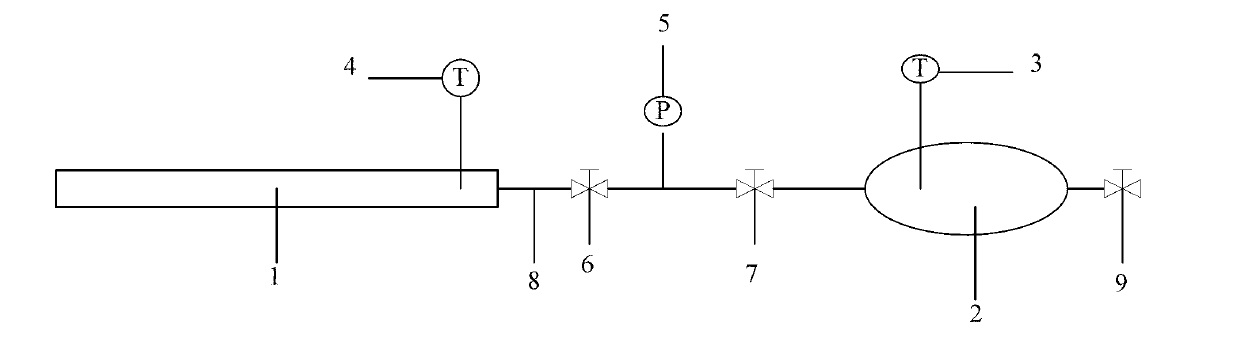
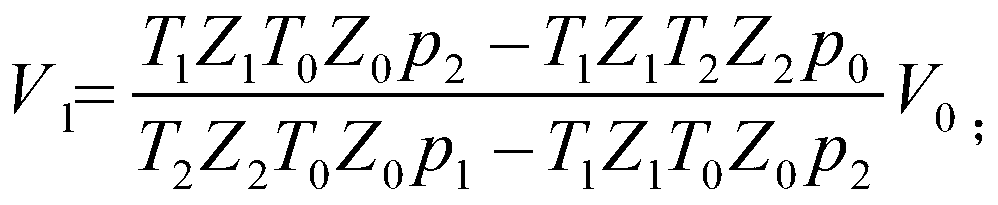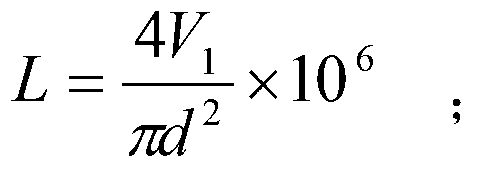Measuring device blocking position of gas pipeline and measuring method thereof
A technology for gas pipelines and measuring devices, applied in gas/liquid distribution and storage, pipeline systems, mechanical equipment, etc., can solve the problems of time-consuming and laborious staff experience, increased production costs, and higher requirements.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] The simulated gas in gas line 1 is methane CH 4 , where the known critical parameter of methane is: p c =4596kPa, T c =190.53K;
[0135] The initial pressure value p of the methane gas in the gas pipeline is obtained by measuring the pressure sensor 5 1 200.0kPa;
[0136] At room temperature 20°C, with inner diameter d=80mm, L s =9.10m seamless steel pipe is the gas pipeline 1, with V 0 =0.11m 3 The elliptical seamless steel tank is a gas tank 2 with an exhaust pressure-resistant valve 9 on the gas tank 2. The air in the gas tank 2 is replaced with the methane CH in the gas line 1 4 out;
[0137] Close the exhaust pressure-resistant valve 9, measure the initial pressure value p of the methane gas in the gas tank 2 with the pressure sensor 5 with an accuracy of 0.1kPa 0 , measure the gas initial temperature T in the gas tank 2 with the first Pt thermometer 3 0 , the initial pressure value p of methane in the gas tank 2 is measured 0 =100kPa; the temperature me...
Embodiment 2
[0149] The simulated gas in gas line 1 is methane CH 4 , where the known critical parameter of methane is: p c =4596kPa, T c =190.53K;
[0150] The initial pressure value p is obtained by measuring the pressure sensor 5 1 8000.0kPa;
[0151] At room temperature 20°C, with inner diameter d=80mm, L s =9.10m seamless steel pipe as the gas pipeline, with V 0 =0.11m 3 The elliptical seamless steel tank is a gas tank 2, and the gas tank 2 has an exhaust pressure-resistant valve 9. The air in the gas tank 2 is replaced with the methane CH in the gas line 1 4 out;
[0152] Close the exhaust pressure-resistant valve 9, and measure the initial pressure value p of the methane gas in the gas tank 2 with the pressure sensor 5 with an accuracy of 0.1kPa 0 , measure the gas initial temperature T in the gas tank 2 with the first Pt thermometer 3 0 , the initial pressure value p of methane in the gas tank 2 is measured 0 =100kPa, the measured temperature in the gas tank 2 is room te...
Embodiment 3
[0162] The simulated gas in gas line 1 is carbon dioxide CO 2 , where the known critical parameter of carbon dioxide is: p c =7375kPa, T c =304.13K;
[0163] The initial pressure value p of carbon dioxide gas in the gas pipeline is obtained by measuring the pressure sensor 5 1 200.0kPa;
[0164] At room temperature 20°C, with inner diameter d=80mm, L s =9.10m seamless steel pipe is simulated gas pipeline 1, with V 0 =0.11m 3 The elliptical seamless steel tank is a gas tank 2 with an exhaust pressure-resistant valve 9 on the gas tank 2. The air in the gas tank 2 is replaced with the simulated gas carbon dioxide CO 2 out;
[0165] Close the exhaust pressure-resistant valve 9, measure the initial pressure value p of the carbon dioxide gas in the gas tank 2 with the pressure sensor 5 with an accuracy of 0.1kPa 0 , measure the gas initial temperature T in the gas tank 2 with the first Pt thermometer 3 0 , measure the initial pressure value p of carbon dioxide in the gas t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com