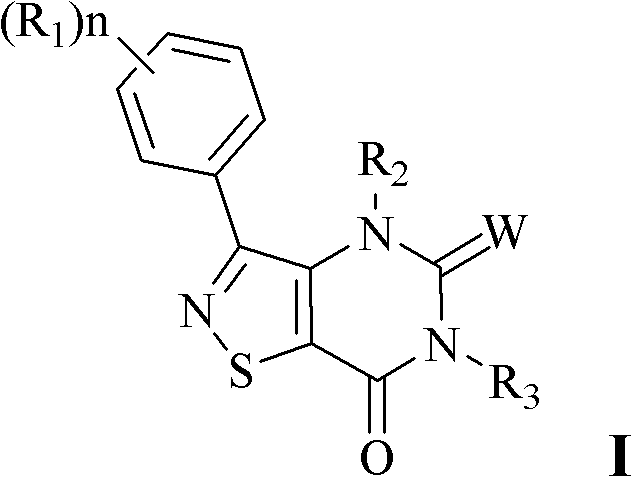
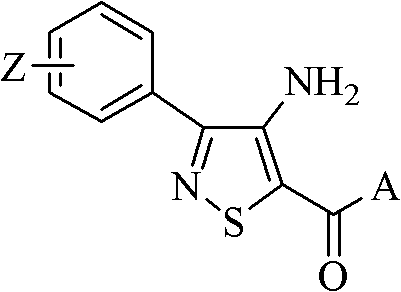
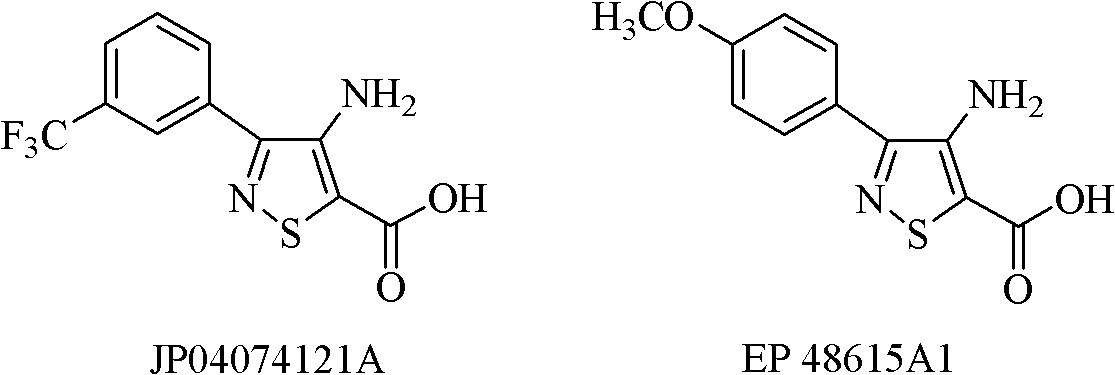
Isothiazole-o-pyrimidone compound and application thereof
A kind of technology of pyrimidinone and compound, applied in the field of isothiazolopyrimidone compound, can solve the problem that structure isothiazolopyrimidone compound has not been reported and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] Example 1: Preparation of compound 160
[0122]
[0123] 1) Preparation of intermediate 4-amino-3-phenyl-5-isothiazole ethyl ester (II-1)
[0124] (1) Preparation of benzoyl nitrile oxime sodium salt
[0125] Weigh 8.15 g (0.20 mol) of sodium hydroxide and 23.41 g (0.20 mol) of benzyl cyanide into a 1000 ml three-necked flask equipped with mechanical stirring, thermometer and exhaust gas absorption device, add 150 ml of absolute ethanol, and cool to 10-20°C. At this temperature, a solution of 28.11 g (0.24 mol) of isoamyl nitrite in 50 ml of absolute ethanol was slowly added dropwise. After the addition, the ice bath was removed, and the reaction was continued for 2.5 hours at room temperature. After the reaction was monitored by TLC, 200ml of ether was added to the reaction solution, stirred for 30 minutes and filtered, the filter cake was washed with ether, dried in air and vacuum (70-80℃) to obtain 15.31 g of white solid, melting point: 286-288 ℃.
[0126] (2) Preparatio...
Embodiment 2
[0148] Example 2: 30% compound 7 wettable powder
[0149]
[0150] The compound 7 and other components are fully mixed and pulverized by an ultrafine pulverizer to obtain a 30% wettable powder product.
Embodiment 3
[0151] Example 3: 30% compound 48 wettable powder
[0152]
[0153] The compound 48 and other components are fully mixed and pulverized by an ultrafine pulverizer to obtain a 30% wettable powder product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com