Melittin antitone analogue and preparation method for same
A melittin anti-analog technology, which is applied in the field of melittin reverse analogs and its preparation, can solve the problems of disappearance of hemolytic activity and limitation of clinical application, and achieve low immunogenicity and good water solubility , the effect of good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
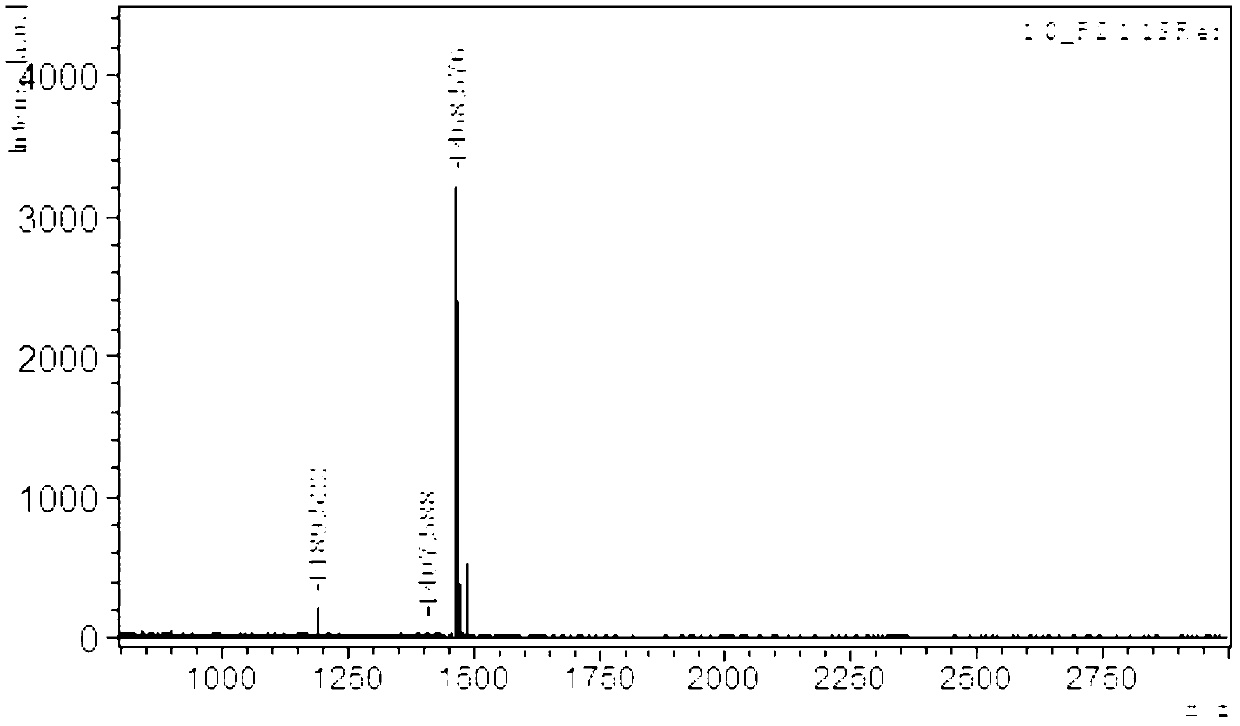
Image
Examples
Embodiment Construction
[0033] 1. Solid-phase chemical peptide synthesis reaction of reverse sequence analogue of melittin
[0034] We designed and synthesized a series of reverse peptide analogs based on Mel(12-26) with different numbers of basic amino acid residues and different chain lengths of hydrophobic fragments. Bacterial activity is very important, and the N-terminal retains at least 3 basic amino acids (positive charge > 4) and the C-terminal hydrophobic segment with a chain length of at least 8 amino acid residues has higher antibacterial activity. The hemolytic activity of sequence peptides is very small.
[0035] According to the core functional sequence of Melittin, the reverse sequence of Melittin (12-24): RKRKIWSILAPLG-COOH in the fifth position of I is replaced by R, and a basic amino acid is added to further enhance the positive charge. At the same time, the 8 hydrophobic amino acids at the C-terminal remain unchanged. The amino acid sequence is: RKRKRWSILAPLG-COOH. The N-terminal ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com