Method for producing isoindoline yellow pigment
A technology of indoline yellow and a production method, applied in the directions of organic dyes, organic chemistry, etc., can solve the problems of high recovery cost, difficulty in recycling and high price, and achieve the effects of low production cost, convenient industrial production and little environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
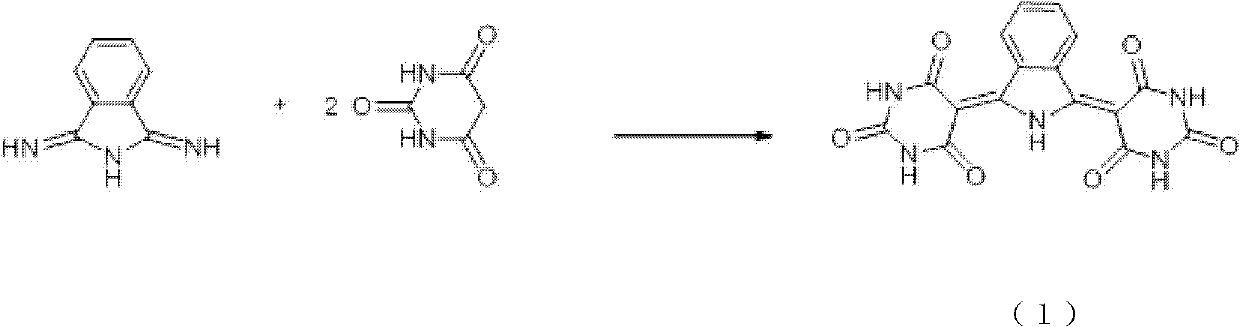
[0018] In reactor, add 3717 grams of water, 1237g weight concentration is 20% hydrochloric acid, then add 256g (2 mole) barbituric acid and 145g (1 mole) 1,3-diiminoisoindoline, be warming up to 70 ℃, stirred and reacted for 15 hours, filtered, washed with 3000 g of hot water at 55 ℃ at 85 ℃, and dried at 110 ℃ for 10 hours to obtain 348.6 g of Pigment Yellow PY139 with a yield of 95%.
[0019] Wherein: the weight concentration of hydrochloric acid aqueous solution is 4.99%; The weight of hydrochloric acid is 0.97 times of barbituric acid.
Embodiment 2
[0021] In reactor, add 3717 grams of water, 294g weight concentration is 20% hydrochloric acid, then add 256g (2 mole) barbituric acid and 145g (1 mole) 1,3-diiminoisoindoline, be warming up to 60 °C, stirred for 10 h, filtered, washed with 3000 g of hot water at 8550 °C, and dried at 110 °C for 10 h to obtain 348.6 g of Pigment Yellow PY139 with a yield of 90%.
[0022] Wherein the weight concentration of hydrochloric acid aqueous solution is 1.47%; The weight of hydrochloric acid is 0.23 times of barbituric acid.
Embodiment 3
[0024] Substitute 1237g of 20% hydrochloric acid with 539g of 20% hydrochloric acid by weight concentration, repeat Example 1, and the yield is 92%.
[0025] Wherein the weight concentration of hydrochloric acid aqueous solution is 2.69%; The weight of hydrochloric acid is 0.23 times of barbituric acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com