Nasal spray of seaweed extract and preparation method of nasal spray
A technology of seaweed extract and nasal spray, which is applied in the directions of medical preparations, antiviral agents, and pharmaceutical formulations containing active ingredients, and achieves the effects of convenient use, simple operation and obvious effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Preparation of seaweed extract nasal spray
[0022] Prescription composition: seaweed polysaccharide 2g, glycerin 200g, sodium chloride 50g, and the rest is water for injection.
[0023] Preparation process: Take the prescribed amount of seaweed polysaccharide and add it into a sterilized container, add an appropriate amount of 300ml of sterilized water for injection treated with activated carbon, stir evenly until clear liquid, add an appropriate amount of moisturizing agent to dissolve, stir evenly, and then add the remaining After cooling 700 ml of water for injection, adjust the osmotic pressure to 285-310 (mOsm / kgH 2 O), filter, seal the filtrate and heat at 100°C for 30min, cool to room temperature, fill, and distribute in plastic spray bottles (12mL / bottle), to get ready. (Note: The mouth of the bottle is a special spray pump (spray system), including a sterile filter)
Embodiment 2
[0024] Example 2 Nasal spray properties, pH, minimum filling volume, and inspection of each spray volume
[0025] According to the preparation method of the embodiment, three batches of products were prepared, respectively 001, 002, and 003 batches, and their properties, pH value, filling volume, per spray volume, and uniformity were inspected.
[0026] Table 1 Inspection results
[0027] batch character PH value Loading ml Each injection volume μl / time 001 Transparent colorless viscous liquid 7.01 11.98±0.19 116.3±1.8 002 Transparent colorless viscous liquid 7.10 12.01±0.16 108.6±1.4 003 Transparent colorless viscous liquid 7.05 11.98±0.29 112.7±1.6
[0028] The nasal spray is a transparent, colorless liquid with a uniform solution and a neutral pH, and each spray dose contains a uniform amount of the main drug.
Embodiment 3
[0029] Example 3 Study on Stability of Seaweed Polysaccharide Nasal Spray
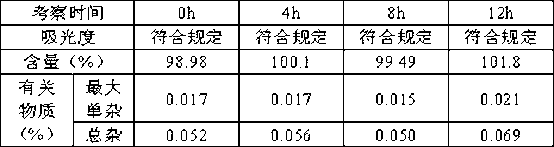
[0030] This product investigates the preparation of the solution under normal light conditions. The solution is placed under normal light conditions for 12 hours, and the absorbance, content and related substances are measured at 0, 4, 8 and 12 hours. The results are as follows:
[0031] The solution stability result prepared under the normal light condition of table 2
[0032]
[0033] It can be seen from the experimental results that the color, content and related substances of the seaweed polysaccharide nasal spray solution prepared under normal light conditions meet the requirements, and compared with 0h after being placed under normal light conditions for 12 hours, there is no obvious change in absorbance, content and related substances. Therefore, under normal light conditions, the prepared seaweed polysaccharide solution is stable.
[0034] Strong light exposure test Place the product hori...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com