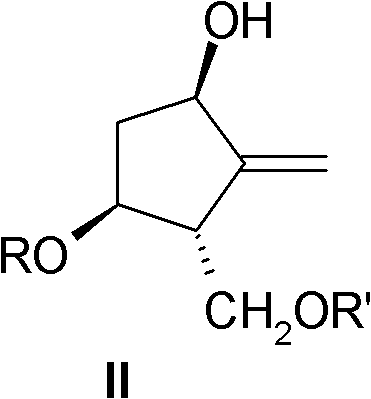
Synthesis intermediate of Entecavir and preparation method thereof
A compound and selected technology, applied in the preparation of hydroxyl compounds, organic compounds, chemical instruments and methods, etc., can solve the problems of harsh reaction conditions, large environmental pollution, and high equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
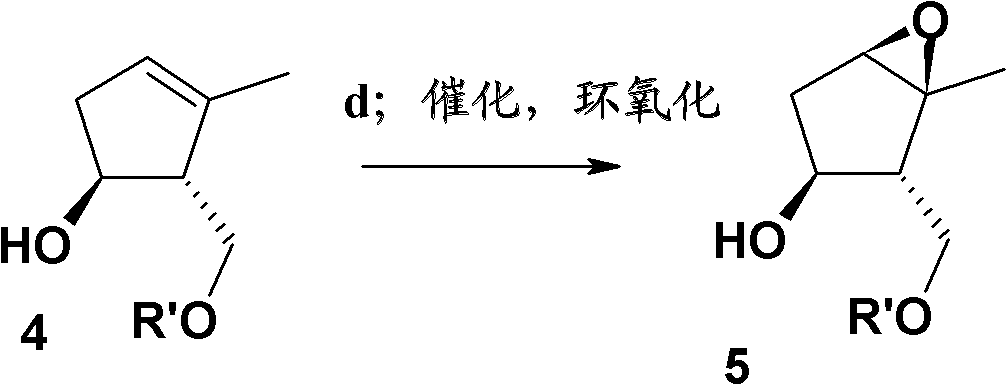
Image
Examples
Embodiment 1
[0209] Example 1: Preparation of (2R, 3S)-2-(prop-1-en-2-yl)hex-5-ene-1,3-diol (compound 2)
[0210]
[0211] Suspend 1.0g CuI (5mmol) in 50ml of anhydrous ether, cool to -20°C, add isopropene Grignard reagent 0.5M 100ml THF solution (50mmol) dropwise under the protection of argon, and stir at -20°C for 25min , cooled to -78°C, added dropwise a 15ml THF solution containing 1.6g (14.2mmol) of compound 1, heated to -20°C and stirred for 16 hours, then added saturated NH 4 Cl 200ml, the organic layer was separated, and the aqueous layer was extracted with EtOAc 100ml x 2. The organic layers were combined, washed with water, and evaporated to dryness to obtain 2.63g of an oily substance. Add 20ml THF, 30ml H 2 O, add 3.0g NaIO under stirring 4 , stirred at room temperature for 3h. Saturated with Na 2 SO 3 , separate the organic layer, extract the aqueous layer with EtOAc 20ml x 2, combine the extracts, wash with saturated NaCl, wash with anhydrous NaCl 2 SO 4 After dryin...
Embodiment 2
[0215] Example 2: Preparation of (3R, 4S)-3-((tert-butyldimethylsilyloxy)methyl)-2-methylhept-1,6-dien-4-ol (compound 3, wherein R'=t-Bu(Me) 2 Si-)
[0216]
[0217] Add 232mg of 2 to 7.5ml CH 2 Cl 2 Dissolve, add 202mg of imidazole, cool to 0°C, add 245mg of TBSCl under stirring, and add 0°C to room temperature for 10 hours. Add 10ml of water, separate the organic layer, wash with water, anhydrous Na 2 SO 4 It was dried, filtered and purified by a short column of silica gel eluting with petroleum ether / EtOAc (50 / 1) to give the product 290 mg (72.3%), Rf=0.75 (petroleum ether / EtOAc (20 / 1)).
[0218] [α] D 20 =-25 (c 0.5, CHCl 3 );
[0219] 1 H NMR (300M, CDCl 3 , ppm) δ5.98-5.86 (m, 1H), 5.12 (d, 1H, J=6.3Hz), 5.07 (s, 1H), 4.86 (s, 1H), 4.76 (s, 1H), 3.94-3.77 (m, 3H), 2.09-2.35(m, 3H), 1.71(s, 3H), 0.90(s, 9H), 0.08(s, 6H);
[0220] 13 C NMR (100M, CDCl 3 , ppm) δ143.6, 135.5, 116.9, 113.4, 74.0, 66.9, 53.3, 39.8, 25.8, 22.0, 18.1.
[0221] HR-MS (ESI) cal...
Embodiment 3
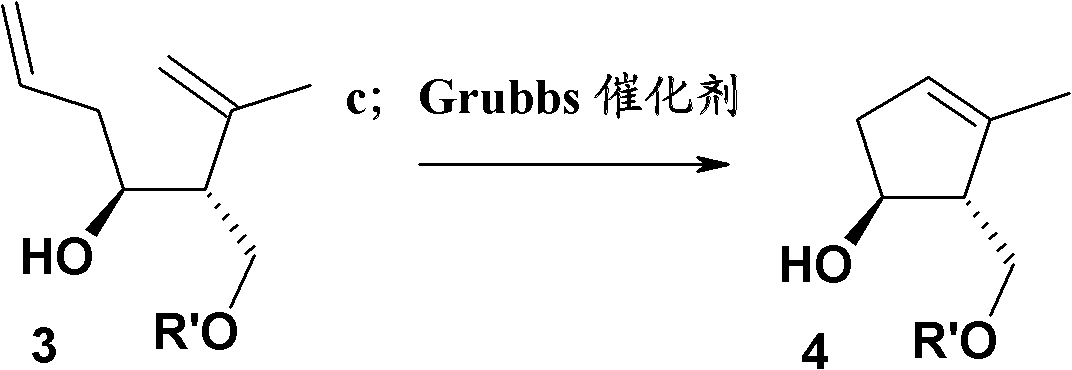
[0222] Example 3: Preparation of (1S, 2R)-2-((tert-butyldimethylsilyloxy)methyl)-3-methylcyclopent-3-en-1-ol (compound 4, wherein R' =t-Bu(Me) 2 Si-)
[0223]
[0224] 820 mg of compound 3 was dissolved in 20 ml of dichloromethane, and 50 mg of Grubbs second-generation catalyst (2%) was added in an argon atmosphere, heated to reflux and stirred for 2 hours in an argon atmosphere, concentrated under reduced pressure, and the residue was purified by a short silica gel column, petroleum ether / Elution with EtOAc (20 / 1) gave 600 mg (81.5%) of the product, Rf = 0.35 (petroleum ether / EtOAc (10 / 1)).
[0225] [α] D 20 =+34 (c 0.25, CHCl 3 );
[0226] 1 H NMR (300M, CDCl 3 , ppm) δ5.32 (s, 1H), 4.34-4.29 (m, 1H), 3.85 (dd, 1H, J=9.9, 4.8Hz) 3.46 (t, J=8.4Hz), 2.64-2.56 (m, 2H), 2.21-2.16(m, 1H), 1.66(s, 3H), 0.9(s, 9H), 0.10(s, 6H).
[0227] 13 C NMR (125M, CDCl 3 , ppm) δ138.1, 123.4, 64.1, 59.2, 39.9, 25.9, 18.2, 15.3, -5.5, -5.5.
[0228]HR-MS (ESI) calculated value C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com