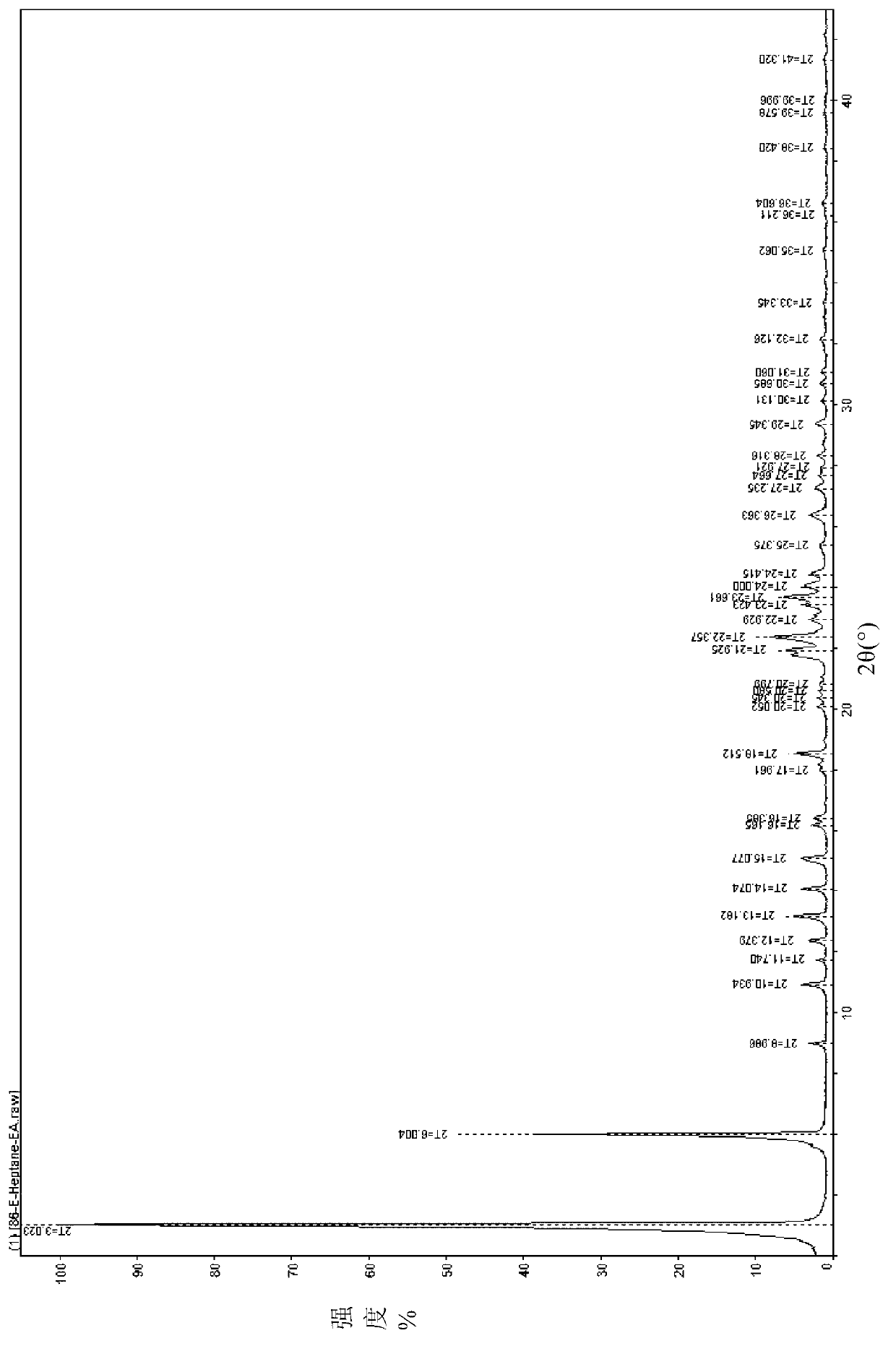
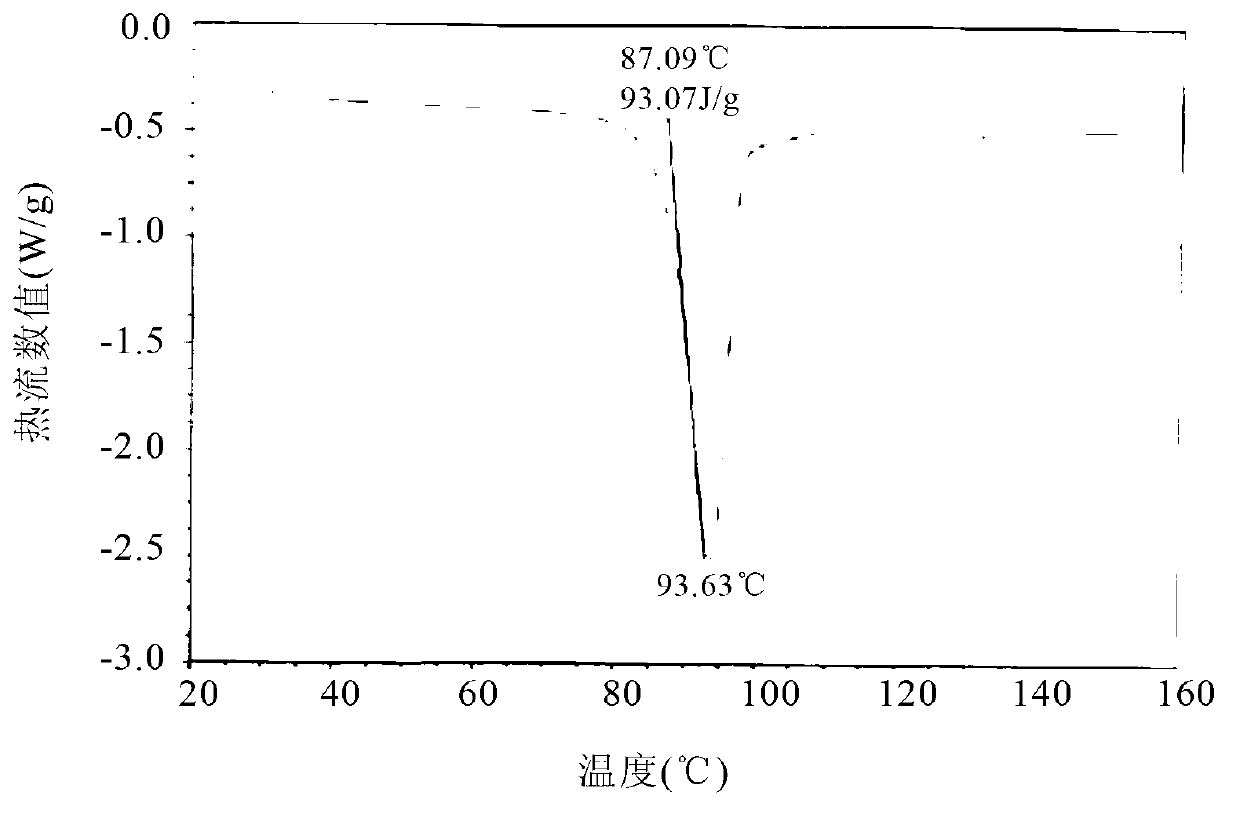
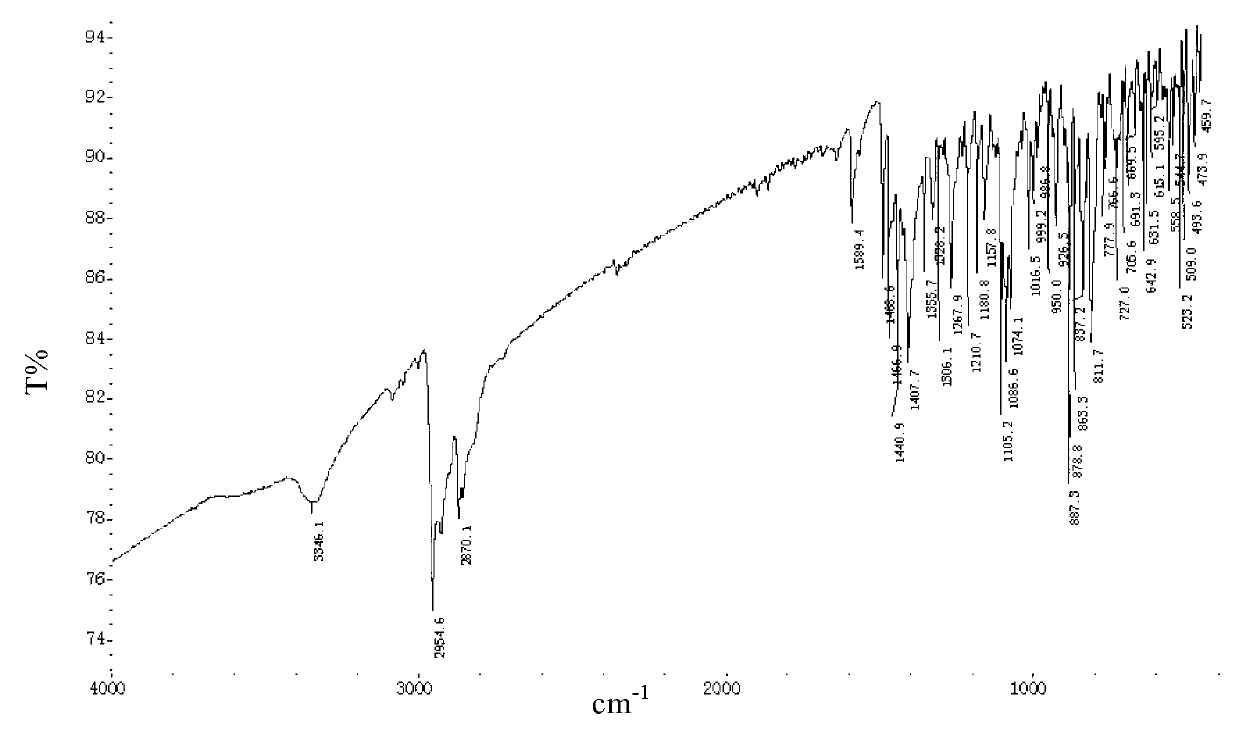
Polymorphic substances of E-type lumefantrine and preparation methods thereof
A technology of polymorphs and crystal forms, which is applied in the preparation of organic compounds, the preparation of aminohydroxyl compounds, chemical instruments and methods, etc., can solve the problems of long melting range, decreased yield of E formula, low melting point of products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0079] 1. Crystal of (9E)-2,7-dichloro-9-[(4-chlorophenyl)methylene]-α-[(di-n-butylamino)methyl]-9H-fluorene-4-methanol The preparation method of the polymorph of type I, comprises the steps:
[0080] (1) Provide (9E)-2,7-dichloro-9-[(4-chlorophenyl)methylene]-α-[(di-n-butylamino)methyl]-9H dissolved in an inert solvent - a solution of fluorene-4-methanol, the (9E)-2,7-dichloro-9-[(4-chlorophenyl)methylene]-α-[(di-n-butylamino)methyl]- The chemical purity of 9H-fluorene-4-methanol is ≥95%;
[0081] The solution is prepared by dissolving benzfluorenol containing E-type benzfluorenol in an inert solvent; preferably, the weight-volume ratio of benzfluorenol to the inert solvent is 1g:3~300ml; preferably 1g:3 ~30ml.
[0082] Wherein, the inert solvent is selected from the group consisting of ketone solvents, ester solvents, aromatic hydrocarbon solvents, ether solvents, or mixtures thereof;
[0083] The ketone solvent is selected from the group consisting of acetone, butanone,...
Embodiment 1
[0127] Example 1 Preparation of (9E)-2,7-dichloro-9-[(4-chlorophenyl)methylene]-α-[(di-n-butylamino)methyl]-9H-fluorene-4-methanol (E isomer)
[0128] Under the protection of nitrogen, add NaOH (7.0g, 0.18mol), 200ml of absolute ethanol, and 40ml of toluene into the reaction flask, stir and dissolve at 25°C, add α-(di-n-butylaminomethyl)-2,7- Dichloro-4-fluorenylmethanol (20.0 g, 0.049 mol) was added to p-chlorobenzaldehyde (8.0 g, 0.057 mol) g, and the reaction was kept under nitrogen atmosphere and stirred at 25°C for 3 hours. After the reaction was complete as detected by TLC, 1N hydrochloric acid ethanol solution was added to adjust the pH of the reaction solution to 7. The reaction mixture was concentrated to dryness under reduced pressure. The concentrate was heated to 65° C. with 200 ml of toluene and stirred to dissolve, the organic phase was washed with water until the pH value of the aqueous phase was 6.5, the organic phase was separated, and concentrated to drynes...
Embodiment 2
[0134] Example 2 Preparation of (9E)-2,7-dichloro-9-[(4-chlorophenyl)methylene]-α-[(di-n-butylamino)methyl]-9H-fluorene-4-methanol Form I
[0135] 5.0g (9E)-2,7-dichloro-9-[(4-chlorophenyl)methylene]-α-[(di-n-butylamino)methyl]-9H-fluorene-4-methanol, 20ml of ethyl acetate was added into the reaction flask, stirred and dissolved at room temperature under nitrogen atmosphere. Cool down to 0°C for crystallization. After filtering, the filter cake was dried to dryness under reduced pressure at 50° C. to obtain 3.2 g of needle crystals.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com