Anti-malarial medicinal composition and preparation method and application thereof
A composition and anti-malarial technology, which is applied in the direction of drug combination, anti-infective drugs, and pharmaceutical formulations, can solve the problems of insufficient stability of compound solid preparations, low absorption rate of active components, and potential safety hazards, so as to facilitate absorption , release behavior is good, and the effect of ensuring safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The preparation of embodiment 1 pharmaceutical composition of the present invention
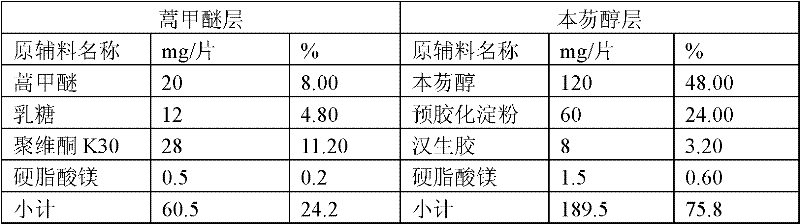
[0046] Take the raw and auxiliary materials according to the weight ratio stated in Table 1:
[0047] Table 1
[0048]
[0049] The raw and auxiliary materials used in the present invention can all be obtained by purchasing commercially available products, wherein, the purity of artemether and bemefluorenol is determined to be 98-102%.
[0050] Steps:
[0051] 1. The preparation method of artemether layer granules: mix artemether, lactose, and 80% povidone K30, and dissolve the remaining 20% povidone K30 with an appropriate amount of water to make an aqueous solution, which is used as a binder for granulation. Made into soft materials, made into granules by a swinging granulator, dried at 50-60°C, granulated with a 20-mesh sieve, and set aside;
[0052] 2. The production method of benzfluorenol layer granules: mix benzfluorenol and pregelatinized starch, add appropriate amount ...
Embodiment 2
[0055] Embodiment 2 The screening of pharmaceutical composition prescription of the present invention
[0056] Because artemether and lumefantrine may explode during the mixing process, the present invention prepares the compound of artemether and lumefantrine into a double-layer tablet to avoid the mixing of the two, thereby avoiding the occurrence of accidents. Because artemether and lumefantrine have low solubility in water, it is very necessary to have a high requirement for dissolution rate when they are prepared into tablets. In this screening experiment, the type and dosage of the excipients of the pharmaceutical composition of the present invention were screened mainly by taking the disintegration time limit and dissolution rate of the tablet as indicators.
[0057] No. 1 preparation prescription: see embodiment 1.
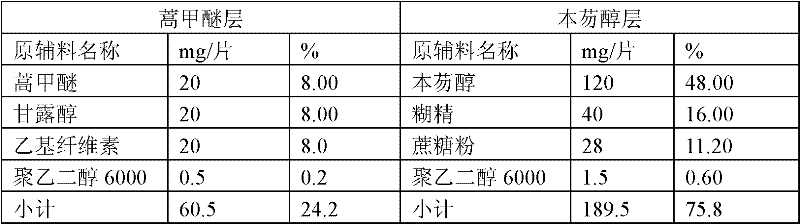
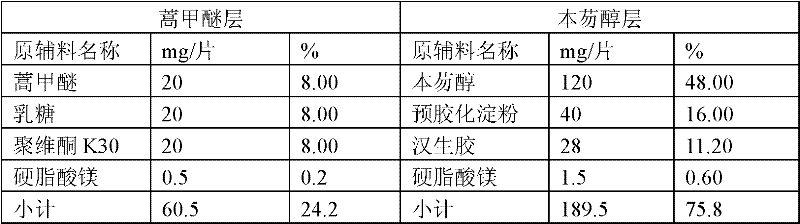
[0058] Prescription of preparation No. 2:
[0059] Table 2
[0060]
[0061] Steps:
[0062]1. The production method of artemether layer granules: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com