Preparation method of aromatic hydrocarbon sulfonyl chloride derivative
A technology of aromatic hydrocarbon sulfonyl chloride derivatives and aromatic hydrocarbons, which is applied in the field of preparation of aromatic hydrocarbon sulfonyl chloride derivatives, can solve the problems of inability to directly recycle, excessive demand for chlorosulfonic acid, and high energy consumption, so as to improve utilization value and reduce energy consumption , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
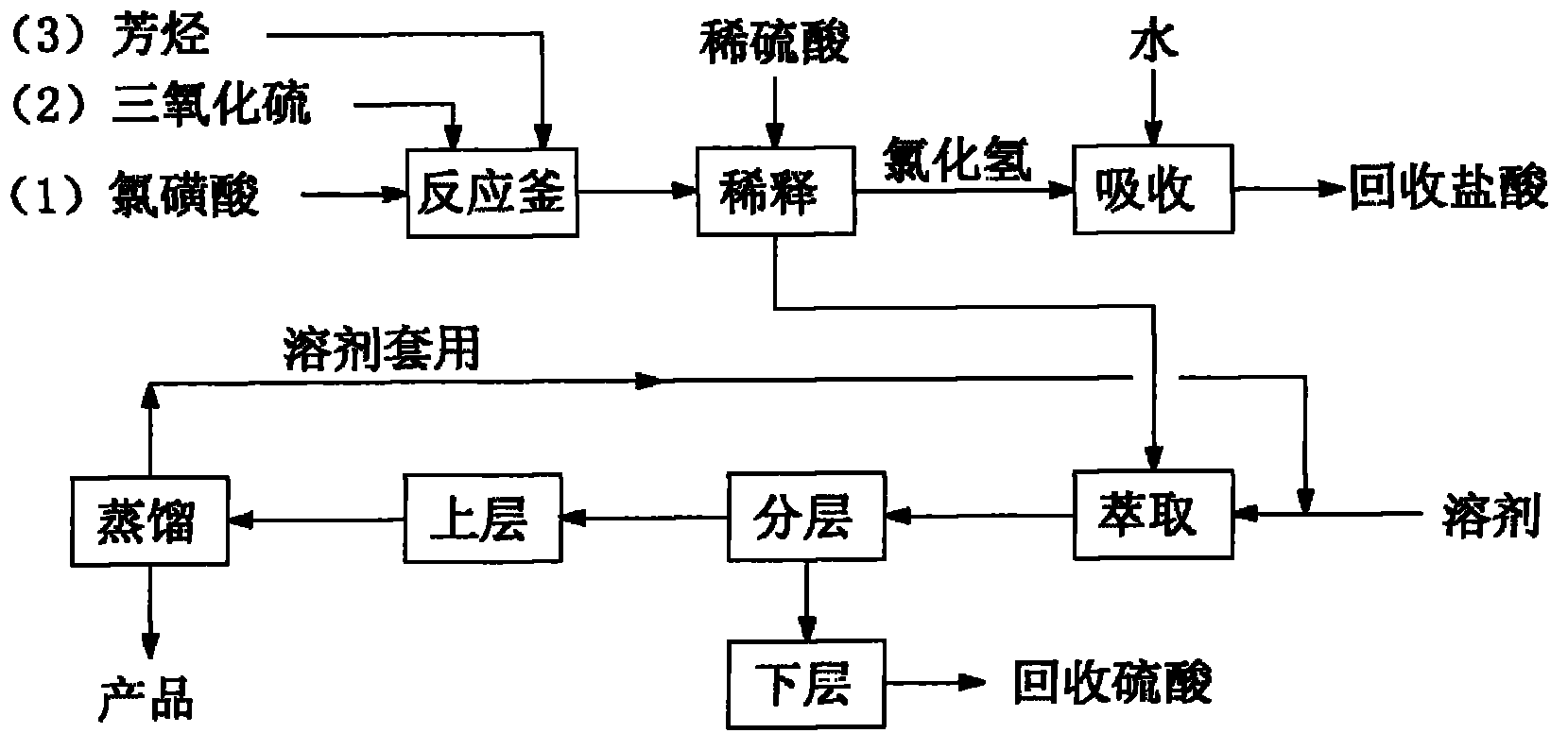
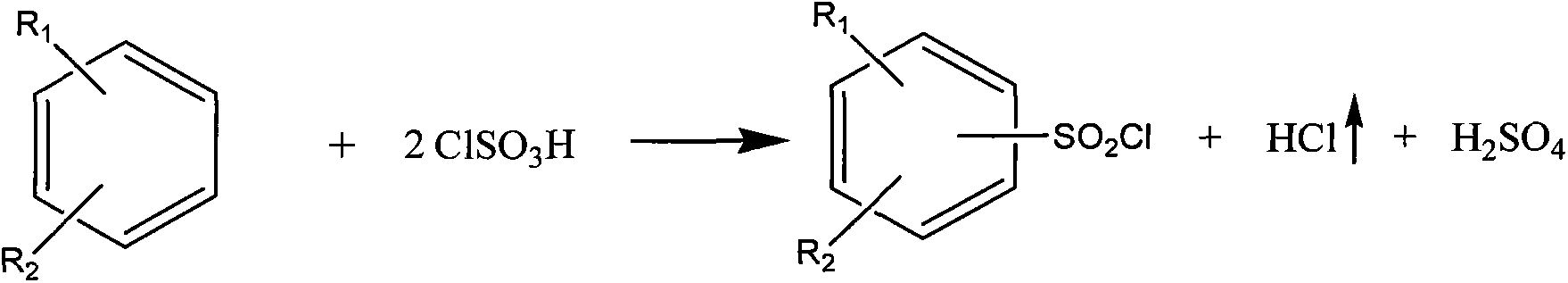
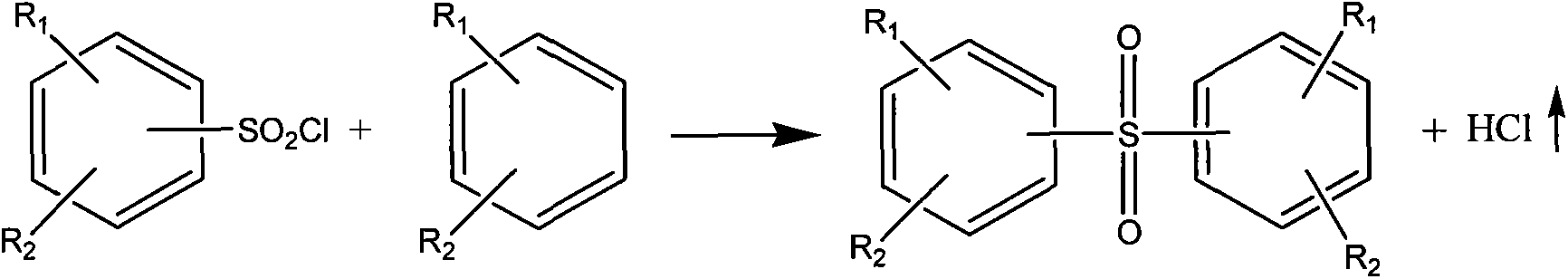
[0036] Add 466g (4mol) of chlorosulfonic acid in a 1000ml flask, feed 40g (0.5mol) of sulfur trioxide gas to make a chlorosulfonic acid solution of sulfur trioxide, then slowly add 123g (1mol) of nitrobenzene , be warming up to 120°C and keep warm until the reaction ends, then cool to 25°C, control the temperature below 40°C, slowly add 448g of 40% by weight dilute sulfuric acid dropwise in the above-mentioned reaction solution, the hydrogen chloride gas generated passes into water, passes through water Can be used as by-product hydrochloric acid recovery after absorbing, finally add the dichloromethane of 12 times of nitrobenzene weight, leave standstill layering after stirring for 1 hour, lower layer is 70% by weight sulfuric acid (hydrochloric acid content is lower than 0.45% by weight), can be used as by-product The product was recovered, and the upper layer was distilled to remove the solvent to obtain 218g of m-nitrobenzenesulfonyl chloride, the content was 99.57%, and th...
Embodiment 2
[0038] Add 291.3g (2.5mol) of chlorosulfonic acid in a 1000ml flask, feed 120g (1.5mol) of sulfur trioxide gas to make a chlorosulfonic acid solution of sulfur trioxide, then slowly add 123g (1mol) of nitric acid Base benzene, heat up to 130°C and keep warm until the reaction is over, then cool to 25°C, control the temperature below 40°C, slowly add 438g of 60% dilute sulfuric acid dropwise to the above reaction solution, the hydrogen chloride gas generated is passed into water, After the water is absorbed, it can be recovered as by-product hydrochloric acid. Finally, ethylene dichloride with 8 times the weight of nitrobenzene is added, and after stirring for 1 hour, it is allowed to stand for stratification. The lower floor is 80% by weight sulfuric acid (hydrochloric acid content is lower than 0.18% by weight), Recovered as a by-product, the upper layer was distilled to remove the solvent to obtain 217g of m-nitrobenzenesulfonyl chloride, the content was 99.05%, and the yield...
Embodiment 3
[0040] Add 349.5g (3mol) of chlorosulfonic acid in a 1000ml flask, feed 80g (1mol) of sulfur trioxide gas to make a chlorosulfonic acid solution of sulfur trioxide, then slowly add 123g (1mol) of nitrobenzene , be warming up to 130°C and keep warm until the reaction ends, then cool to 25°C, control the temperature below 40°C, slowly add 402g of 50% by weight dilute sulfuric acid dropwise in the above-mentioned reaction solution, the hydrogen chloride gas generated is passed into water, passed through water Can be used as by-product hydrochloric acid recovery after absorbing, finally add the carbon tetrachloride of 10 times of nitrobenzene weight, leave standstill layering after stirring for 1 hour, lower layer is 75% by weight sulfuric acid (hydrochloric acid content is lower than 0.40% by weight), can be used as The by-products were recovered, and the upper layer was distilled to remove the solvent to obtain 217g of m-nitrobenzenesulfonyl chloride, the content was 99.00%, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com