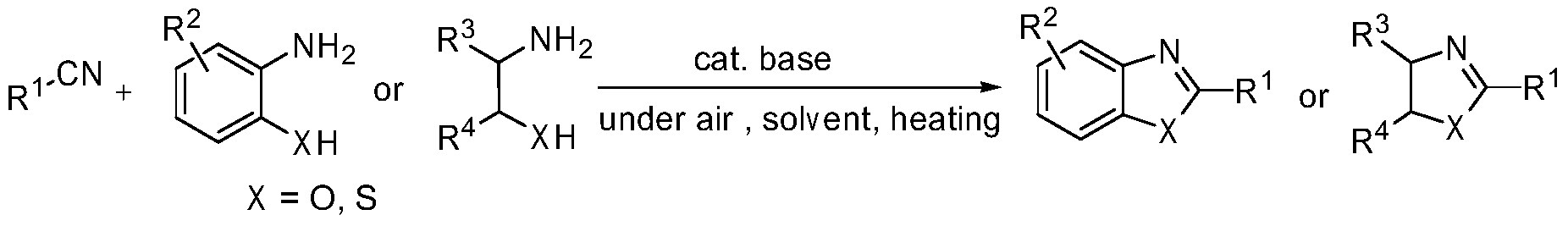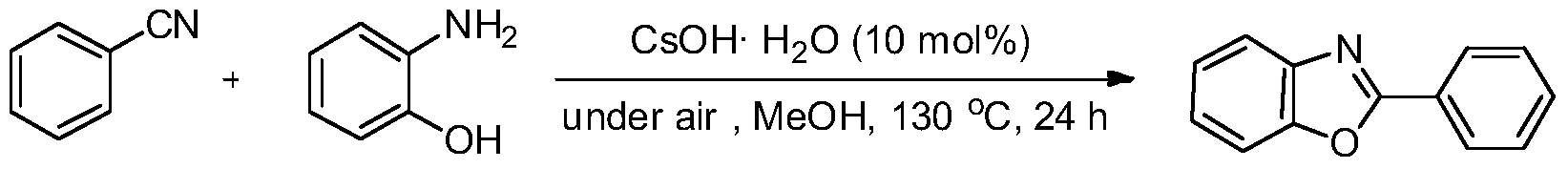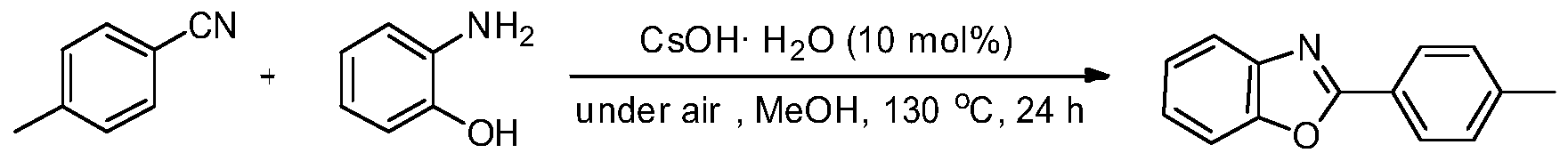Green method for preparing heterocyclic compounds from nitriles
A heterocyclic compound, green technology, applied in the direction of organic chemistry, etc., can solve the problems of large microwave conditions, difficult halogenated compounds, few synthesis methods, etc., and achieves the effect of wide application range, good water solubility and low price.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Preparation of 2-phenylbenzoxazole from benzonitrile and o-aminophenol
[0020]
[0021] Add CsOH·H to the reaction tube sequentially 2 O (0.0168g, 10mol%), PhCN (1.0mmol) and o-aminophenol (0.1309g, 1.2mmol, 1.2equiv.), then add CH 3 OH (0.5mL) was the solvent, and the reaction tube was sealed and heated to 130°C for 24h. The reaction conversion rate was over 96% as measured by GC-MS, and the product was separated and purified by column chromatography with a separation yield of 75%. 1 H NMR (500MHz, CDCl 3 ): δ8.28-8.26(m, 2H), 7.80-7.77(m, 1H), 7.60-7.57(m, 1H), 7.54-7.52(m, 3H), 7.38-7.34(m, 2H). 13 C NMR (125.4MHz, CDCl 3 ): δ163.1, 150.8, 142.1, 131.5, 128.9, 127.6, 127.2, 125.1, 124.6, 120.0, 110.6. MS (EI): m / z (%) 196 (14), 195 (100), 167 (25 ), 166(8), 140(4), 139(4), 103(4), 98(4), 92(12), 84(7), 77(20), 76(8), 64(29 ), 63(44), 62(9), 51(16), 50(10), 39(11), 38(14).
Embodiment 2
[0023] Preparation of 2-(p-methyl)phenylbenzoxazole from p-methylbenzonitrile and o-aminophenol
[0024]
[0025] Add CsOH·H to the reaction tube sequentially 2 O (0.0168g, 10mol%), p-toluonitrile (1.0mmol) and o-aminophenol (0.1309g, 1.2mmol, 1.2equiv.), then add CH 3 OH (0.5mL) was the solvent, and the reaction tube was sealed and heated to 130°C for 24h. The reaction conversion rate was over 89% as measured by GC-MS, and the product was separated and purified by column chromatography with a separation yield of 78%. 1 H NMR (500MHz, CDCl 3 ): δ8.15(d, J=8.5Hz, 2H), 7.77-7.75(m, 1H), 7.59-7.53(m, 1H), 7.35-7.32(m, 4H), 2.44(s, 3H). 13 C NMR (125.4MHz, CDCl 3 ): δ163.3, 150.7, 142.2, 142.1, 129.7, 127.6, 124.9, 124.5, 124.4, 119.9, 110.5, 21.7. MS (EI): m / z (%) 210 (16), 209 (100), 208 (42), 181(6), 180(20), 116(4), 104(6), 92(8), 91(76), 90(9), 89(7), 77(6), 65 (25), 64(8), 63(17), 51(9), 39(12), 38(5).
Embodiment 3
[0027] Preparation of 2-(3-methyl)phenylbenzoxazole from m-methylbenzonitrile and o-aminophenol
[0028]
[0029] Add CsOH·H to the reaction tube sequentially 2 O (0.0168g, 10mol%), m-tolunitrile (1.0mmol) and o-aminophenol (0.1309g, 1.2mmol, 1.2equiv.), then add CH 3 OH (0.5mL) was the solvent, and the reaction tube was sealed and heated to 130°C for 24h. The reaction conversion rate was over 86% as measured by GC-MS, and the product was separated and purified by column chromatography with a separation yield of 79%. 1 H NMR (500MHz, CDCl 3 ): δ8.10(s, 1H), 8.05(d, J=7.5Hz, 1H), 7.79-7.77(m, 1H), 7.59-7.57(m, 1H), 7.42(t, J=7.5Hz, 1H), 7.36-7.34(m, 3H), 2.46(s, 3H). 13 C NMR (125.4MHz, CDCl 3 ): δ163.3, 150.7, 142.1, 138.7, 132.4, 128.8, 128.2, 127.0, 125.0, 124.8, 124.5, 119.9, 110.6, 21.4. MS (EI): m / z (%) 210 (18), 209 ( 100), 208(45), 181(8), 180(50), 179(13), 178(7), 153(5), 152(11), 127(4), 90(8), 89( 6), 77(13), 76(10), 75(5), 63(8).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com