Method for preparing sweet clarithromycin granules
A technology for sweetening clarithromycin and clarithromycin, which is applied in the directions of non-active ingredient medical preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problems of unfavorable product quality control, complicated operation process, etc., Achieve the effect of benefiting human health, controlling product quality, and facilitating production and operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
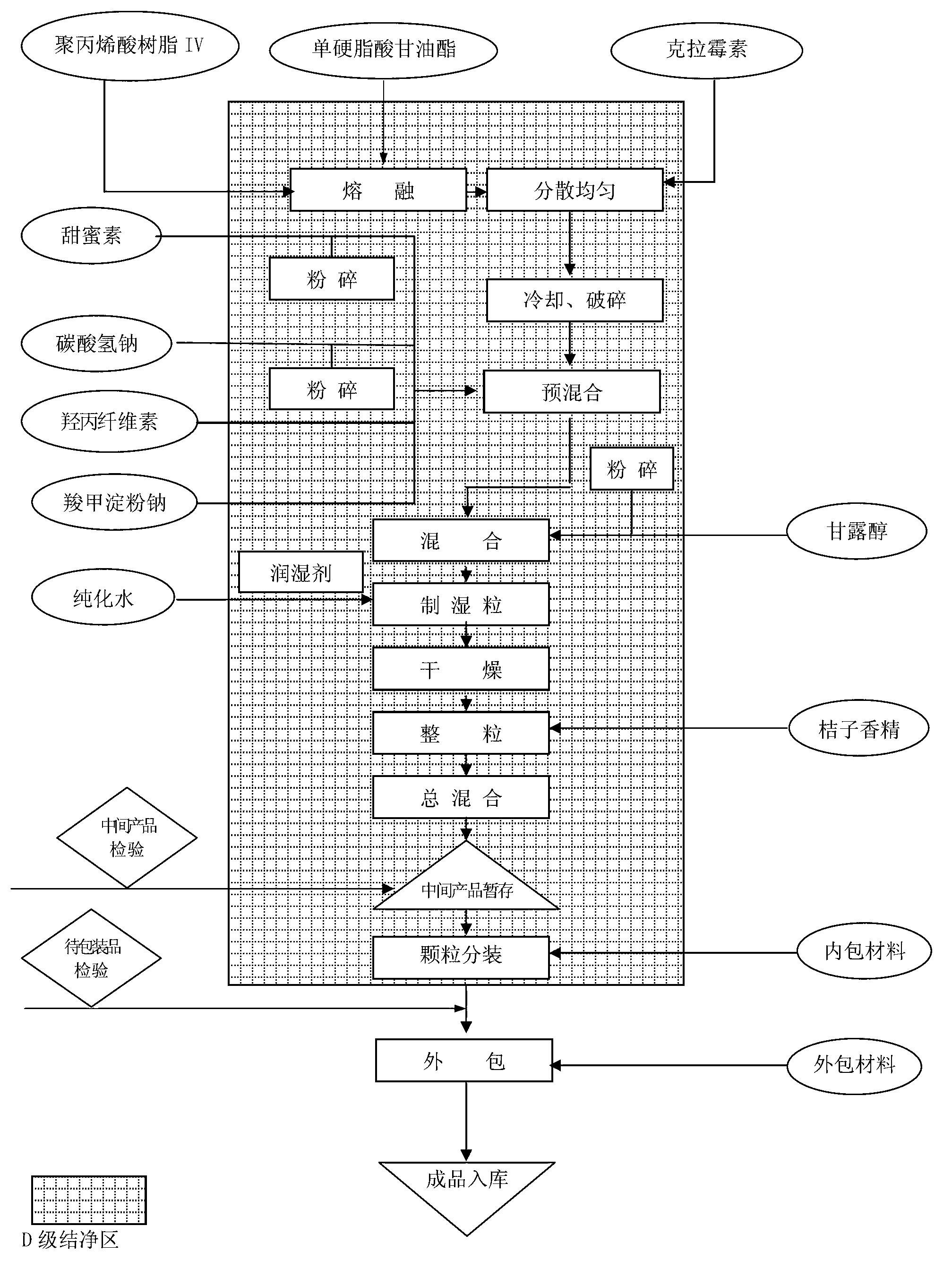
Image
Examples
Embodiment 1
[0026] Take the preparation of 50,000 bags of sweet clarithromycin granules, specifications: 2g:0.125g as an example:
[0027] 1. Pretreatment of raw and auxiliary materials:
[0028] Mannitol, cyclamate, sodium saccharin, and sodium bicarbonate are pulverized separately (using a pulverizer with a screen of Φ0.8mm).
[0029] 2. Weighing materials:
[0030] Weigh each raw and auxiliary material according to the prescription weight:
[0031] Material name
Dosage(kg)
clarithromycin
6.25
12.50
Polyacrylic resin IV
2.50
10.00
0.20
5.00
Cyclamate
7.00
0.50
[0032] purified water
6.40
0.20
55.85
Co-made
100.00
[0033] Remarks: 6.40kg of purified water in the prescription evaporated during the prepar...
Embodiment 2
[0046] Take the preparation of 50,000 bags of sweet clarithromycin granules, specifications: 2g:0.125g as an example:
[0047] 1. Pretreatment of raw and auxiliary materials:
[0048] Mannitol, acesulfame potassium, steviol glycoside, and sodium citrate were pulverized separately (using a pulverizer with a screen of Φ0.8mm).
[0049] 2. Weighing materials:
[0050] Weigh each raw and auxiliary material according to the prescription weight.
[0051] Material name
Dosage(kg)
[0052] clarithromycin
6.25
12.96
Polyacrylic resin IV
2.04
10.00
0.20
5.00
Acesulfame K
6.50
1.00
6.40
lemon zest
0.20
Mannitol
55.85
Co-made
100.00
[0053] Remarks: 6.40kg of purified water in the prescription evaporated during the ...
Embodiment 3
[0066] Take the preparation of 50,000 bags of sweet clarithromycin granules, specifications: 2g:0.125g as an example:
[0067] 1. Pretreatment of raw and auxiliary materials:
[0068] Mannitol, cyclamate, sodium saccharin, and sodium bicarbonate are pulverized separately (using a pulverizer with a screen of Φ0.8mm).
[0069] 2. Weighing materials:
[0070] Weigh each raw and auxiliary material according to the prescription weight.
[0071] Material name
Dosage(kg)
clarithromycin
6.25
12.10
Polyacrylic resin IV
2.90
10.00
0.20
5.00
Cyclamate
7.00
0.50
purified water
6.40
0.20
Mannitol
55.85
Co-made
100.00
[0072] Remarks: 6.40kg of purified water in the prescription evaporated during the preparation process, and 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com