Pharmaceutical composition for treating renal failure in pets
A pharmaceutical composition and kidney failure technology, which is applied in the field of pharmaceutical compositions for pet kidney failure, can solve the problems of high fatality rate, treatment effect and cost to be improved, and achieve the effect of improving recovery rate and good treatment results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0077] Preparation of the composition:
[0078] Prepare liquid A and liquid B containing the following components and contents:
[0079] Solution A: 15g / L glucose, 132mEq / L sodium ion, 96mEq / L chloride ion, 3.5mEq / L calcium ion, 0.5mEq / L magnesium ion, and 40mEq / L lactate ion.
[0080] Solution B: 130mEq / L sodium ion, 109mEq / L chloride ion, 28mEq / L lactate ion, 4mEq / L potassium ion, and 3.0mEq / L calcium ion.
[0081] Detection and evaluation:
[0082] The efficacy of the treatment for renal failure provided by the present invention is evaluated by detecting BUN (blood urea nitrogen) and CRE (creatinine) values and observing the pet's appearance and activity. The BUN and CRE values were detected using a biochemical analyzer (SPOKEN 4430 model) sold by Yusheng Co., Ltd. The BUN and CRE values of dogs and cats in different health states are listed in Table 1 below:
[0083] Table 1
[0084]
[0085] In addition, the pet's health status is assessed by observing the ...
Embodiment 1
[0088] Embodiment 1 (dog) pet name: kiki
[0089] Basic data: pet breed: Maltese dog; gender: female; age: 14 years old; weight 4 kg.
[0090] Health status of animals before administration (Day 0): Acute renal failure caused by inappropriate medication, and the health deteriorated rapidly.
[0091] Therapeutic drugs and methods: A liquid and B liquid are given by subcutaneous injection at the same time, and then (on the third day) are directly administered with A+B mixed solution, and the more doses, the less.
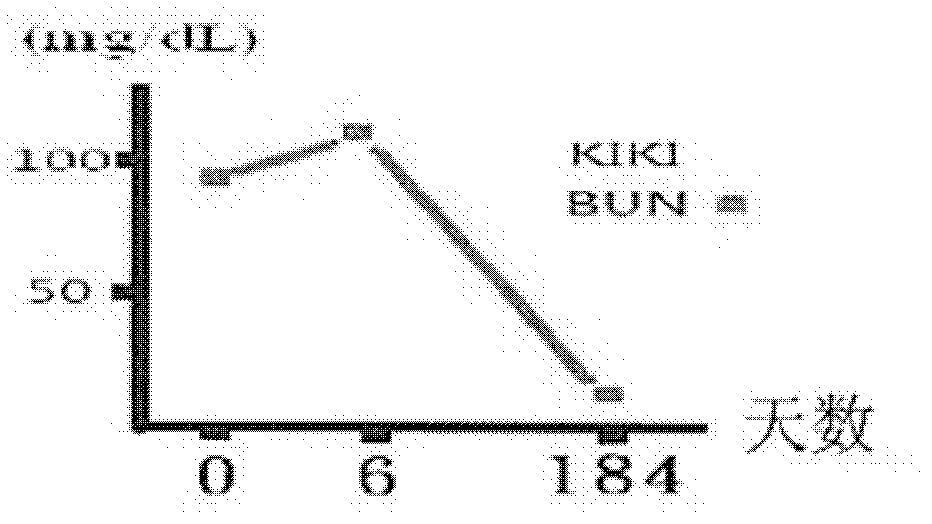
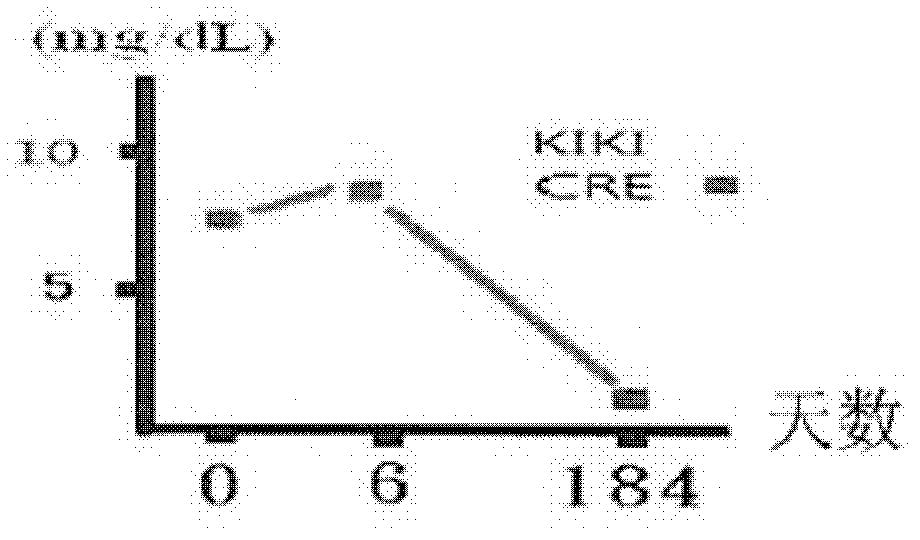
[0092] The therapeutic results after taking the pharmaceutical composition provided by the present invention are shown in the following table 2 and Figure 1a and Figure 1b :
[0093] Table 2
[0094] number of days day 0 day 6 Day 184 Appearance changes Lethargy and vomiting Normal and strong Normal and strong BUN (mg / dL) 92 117 17 CRE (mg / dL) 7.9 8.4 1.1
Embodiment 2
[0095] [0095] Embodiment 2 (dog) pet name: Pipi
[0096] Basic data: pet breed: mixed breed dog; sex: female; age: 6 years old; weight 18 kg.
[0097] Health status of animals before dosing (Day 0): Treatment with steroids resulted in acute renal failure and health deterioration.
[0098] Therapeutic drugs and methods: directly administered with A+B mixture.
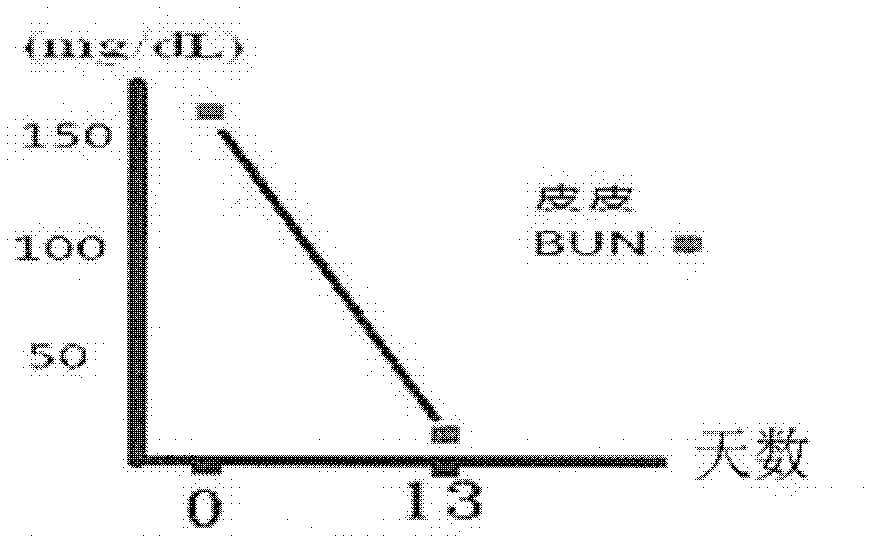
[0099] The therapeutic results after taking the pharmaceutical composition provided by the present invention are shown in the following table 3 and Figure 2a and Figure 2b :
[0100] table 3
[0101] number of days day 0 day 13 Appearance changes bad breath vomiting Normal and strong BUN (mg / dL) 158 18 CRE (mg / dL) 6.7 1.9
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com