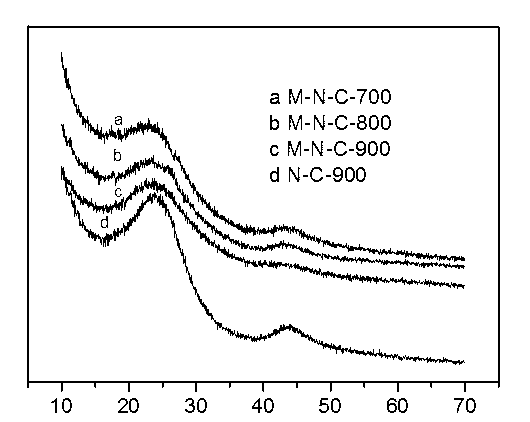
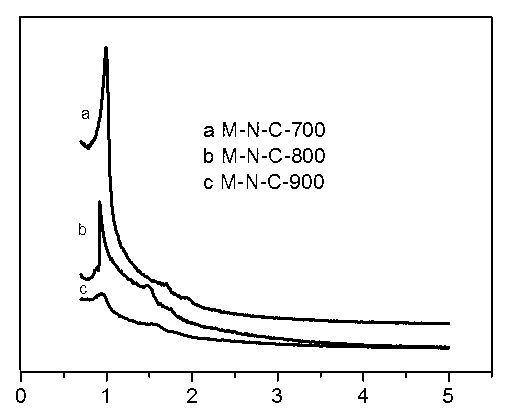
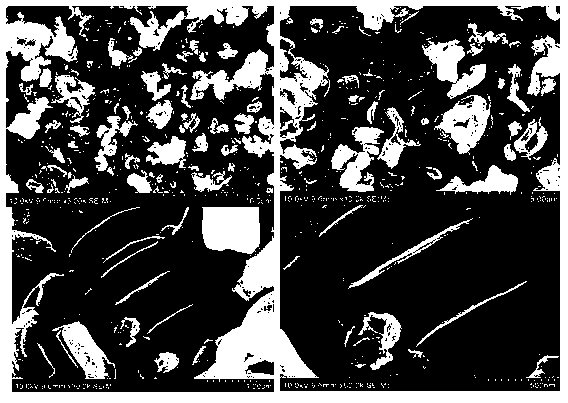
Preparation method of ordered mesoporous non-noble metal-nitrogen-graphitized carbon material
A non-precious metal, graphitized carbon technology, used in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, chemical/physical processes, etc., can solve the problem of poor contact between active sites and conductive carriers, catalytic activity It can reduce the stability of the battery, hinder the output power of the battery, etc., and achieve the effect of increasing the density of catalytic active sites, improving the oxygen conversion rate, superior catalytic stability and methanol resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] a. Synthesis of filling materials
[0036] Dissolve 1 g of bipyridine in 20 mL of absolute ethanol, and after it is completely dissolved, add 0.2018 g of cobalt chloride to the solution to form a metal complex. Then add 0.2g of two-dimensional hard template mesoporous silica SBA-15, and continue to stir until dry; then, dry at a constant temperature at 60°C to obtain the filling material;
[0037] b. High temperature roasting of filling materials
[0038] Place the obtained filling material in a quartz tube, heat it at 500°C and 30ml / min pure nitrogen atmosphere, and keep it for 2 hours to carbonize the filling material to obtain a black powder;
[0039] c. Removal of hard template mesoporous silica
[0040] The black powder prepared above was stirred with 20% hydrofluoric acid solution for 8 hours, washed with deionized water at 60° C., and dried to obtain the ordered mesoporous non-noble metal-nitrogen-graphitized carbon material. The dosage ratio of black powder ...
Embodiment 2
[0042] a. Synthesis of filling materials
[0043] Dissolve 1 g of bipyridine in 20 mL of absolute ethanol, and after it is completely dissolved, add 0.2771 g of cobalt chloride to the solution to form a metal complex. Then add 0.4g of two-dimensional hard template mesoporous silica SBA-15, and continue to stir until dry; then, dry at a constant temperature at 60°C to obtain the filling material;
[0044] b. High temperature roasting of filling materials
[0045]Place the obtained filling material in a quartz tube, heat it at 600°C in a 30ml / min pure nitrogen atmosphere, and keep it for 3 hours to carbonize the filling material to obtain a black powder;
[0046] c. Removal of hard template mesoporous silica
[0047] The black powder prepared above was stirred with 20% hydrofluoric acid solution for 9 hours, washed with deionized water at 60° C., and dried to obtain the ordered mesoporous non-noble metal-nitrogen-graphitized carbon material. The dosage ratio of black powder ...
Embodiment 3
[0049] a. Synthesis of filling materials
[0050] Dissolve 1 g of bipyridine in 20 mL of absolute ethanol, and after it is completely dissolved, add 0.8313 g of cobalt chloride to the solution to form a metal complex. Then add 0.5g of three-dimensional hard template mesoporous silica KIT-6, and continue to stir until dry; then, dry at a constant temperature at 60°C to obtain the filling material;
[0051] b. High temperature roasting of filling materials
[0052] The obtained filling material was placed in a quartz tube, heated at 900°C and 30ml / min pure nitrogen atmosphere, and kept for 5 hours to carbonize the filling material to obtain a black powder;
[0053] c. Removal of hard template mesoporous silica
[0054] The black powder prepared above was stirred with 20% hydrofluoric acid solution for 10 h, washed with deionized water at 60° C., and dried to obtain the ordered mesoporous non-noble metal-nitrogen-graphitized carbon material. The dosage ratio of black powder to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com