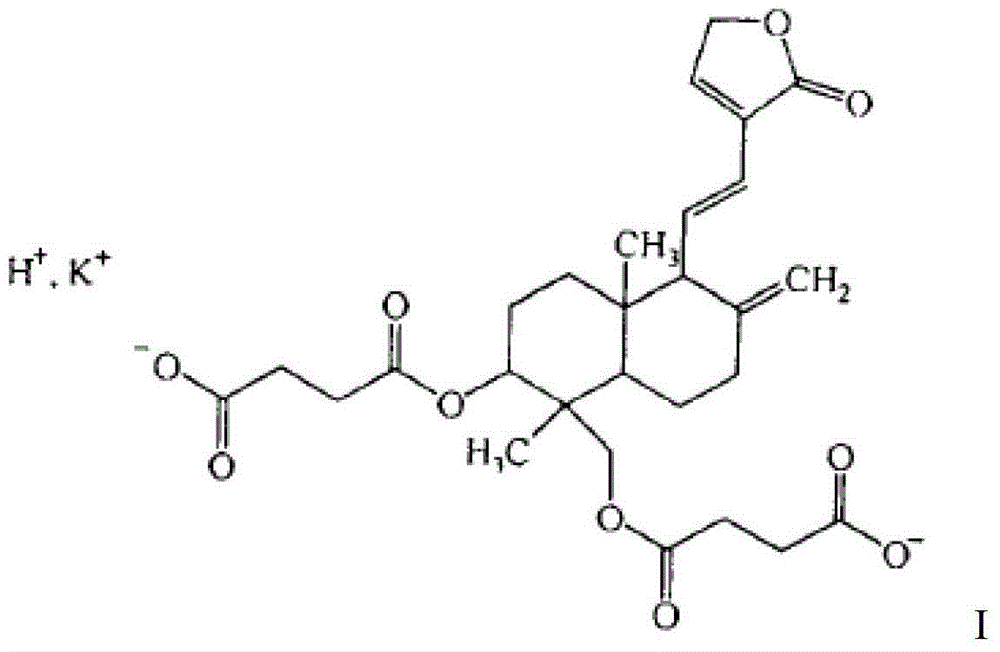
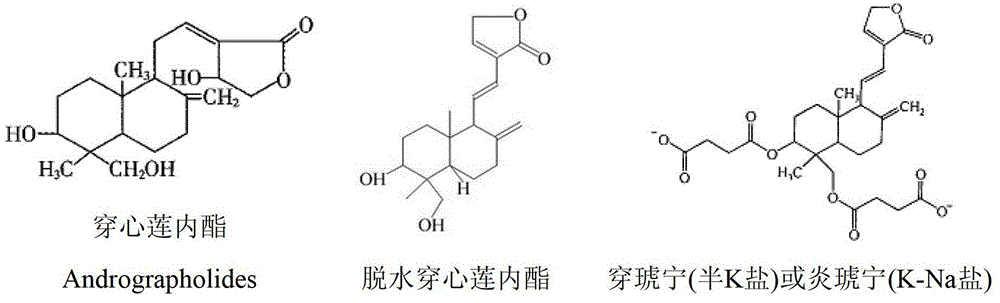
Potassium dehydroandrographolide succinate injection and preparation method
A technology for Chuanhuning and water for injection, which is applied in the field of medicine, can solve the problems of easy hydrolysis of aqueous solutions, decreased content, and deepening of product color and luster.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0086] Preparation Example 1, Preparation of Injection Containing Chuanhuning
[0087] formula:
[0088] Compound of formula I
100mg,
15 mg,
L-cysteine
5 mg,
Hydroxypropyl-β-cyclodextrin
300mg,
Water for Injection
Appropriate amount, add to 5ml.
[0089] Preparation:
[0090] (a) Dissolving alkali metal salts, cysteine or its pharmaceutically acceptable salts, and cyclodextrin in about 85% of the prescribed amount of water for injection;
[0091] (b) Add Chuanhuning to the above solution, stir to dissolve;
[0092](c) Add about 0.3% activated carbon to the solution, stir and adsorb for 30 minutes, filter and decarbonize;
[0093] (d) Add water for injection to the full amount, check the pH value of the solution, and adjust the pH value of the solution to 7.3 with an acid-base regulator if necessary; filter the medicinal solution with 0.4 μm and 0.22 μm microporous membranes, and divide t...
preparation example
[0096] Supplementary preparation example 1: In different prescriptions, except that the amount of sodium bicarbonate is changed to the consumption listed in the second row (mg row) of the following table, other elements in the prescription and the preparation process are all the same as the above-mentioned preparation example 1. Line 1 (No. line) in the table is the obtained injection sample number, the complete number of No. 01 is Eb1-01, which means supplementing No. 01 injection obtained in Preparation Example 1, and the complete number of No. 02 is Eb1-02. similar meaning.
[0097] No.
01
02
03
04
05
06
mg
5
9
12
17
20
25
[0098] Investigate the above samples of Ex1-01 and Eb1-01 to Eb1-06:
[0099] Appearance: Colorless clear liquid.
[0100] Carry out the stability test and test of high temperature treatment according to the above "A, test method example part" method, the result:
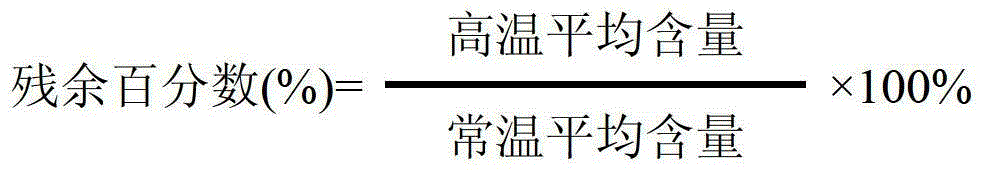
[0101] Conten...
preparation example 2
[0105] Supplementary preparation example 2: In different formulations, except that the amount of cysteine is changed to the amount listed in the second row (mg row) of the following table, other elements and preparation processes in the prescription are the same as the above-mentioned preparation example 1. Line 1 (No. line) in the table is the obtained injection sample number, the complete number of No. 01 is Eb2-01, which means supplementing No. 01 injection obtained in Preparation Example 2, and the complete number of No. 02 is Eb2-02. similar meaning.
[0106] No.
01
02
03
04
05
06
mg
0
1
3
7
10
15
[0107] Investigate the above samples from Eb2-01 to Eb2-06:
[0108] Appearance: Colorless clear liquid.
[0109] Carry out the stability test and test of high temperature treatment according to the above "A, test method example part" method, the result:
[0110] Content residual percentage (%): The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com