Wettable fenbendazole ivermectin powder
A technology of fenbendazole ivermectin powder and fenbendazole, which is applied in the field of wettable fenbendazole ivermectin powder and its preparation, can solve problems such as inconvenient use, and achieve improved dispersibility and preparation The effect of simple process and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation of embodiment 1 wettable fenbendazole ivermectin powder
[0026] Take 4g of fenbendazole, 0.4g of ivermectin, 2g of magnesium stearate, 61g of powdered sugar, 7g of sodium lauryl sulfate, 4g of xanthan gum, 0.8g of fresh milk essence, 0.8g of sodium saccharin, citric acid 20g of sodium, after mixing the above raw and auxiliary materials evenly, pulverize to 200 mesh ultrafine powder, pack, and obtain wettable fenbendazole ivermectin powder of the present invention.
Embodiment 2
[0027] The preparation of embodiment 2 wettable fenbendazole ivermectin powder
[0028] Take 5g of fenbendazole, 0.2g of ivermectin, 2.5g of magnesium stearate, 54.5g of powdered sugar, 9g of sodium lauryl sulfate, 2g of xanthan gum, 0.9g of fresh milk essence, 0.9g of sodium saccharin, Sodium citrate 25g, after the above-mentioned raw and auxiliary materials are mixed uniformly, pulverize to 200 mesh ultrafine powder, pack, obtain the wettable fenbendazole ivermectin powder of the present invention.
Embodiment 3
[0029] Embodiment 3 The compatibility screening of raw and auxiliary materials of wettable fenbendazole ivermectin powder
[0030] (1) Preparation of different formula products
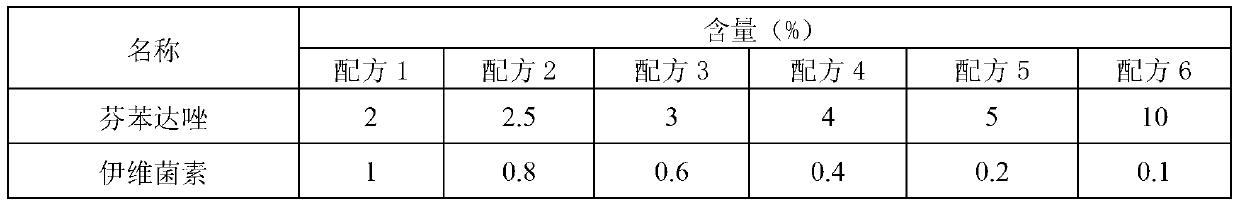
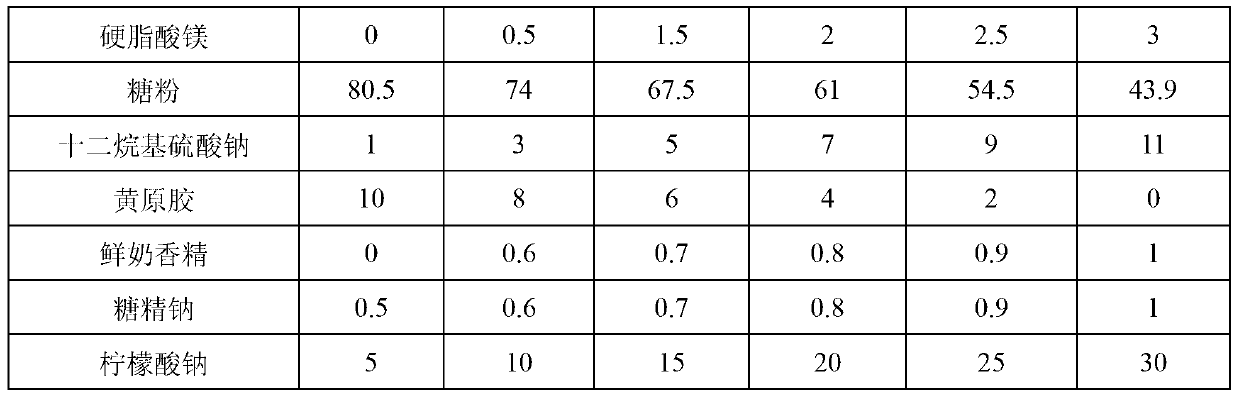
[0031] Get the raw and auxiliary materials in the formula in Table 1, prepare wettable fenbendazole ivermectin powder according to the method of Example 1, and evaluate the pros and cons of each formula.
[0032] Table 1 The preferred scheme of wettable fenbendazole ivermectin powder formulation
[0033]
[0034]
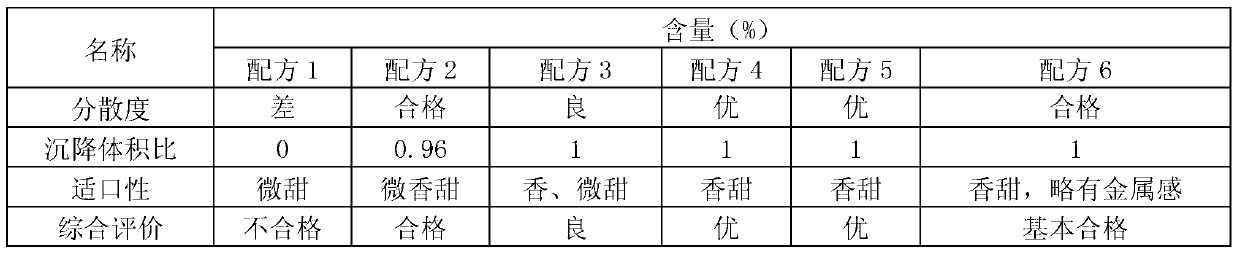
[0035] (2) Quality evaluation of wettable fenbendazole ivermectin powder
[0036] Dispersion evaluation: Take 1 g of each wettable fenbendazole ivermectin powder prepared according to formula 1 to formula 6 in Table 1, put it into 1000 ml of drinking water, observe or stir and observe its dispersion. If the dispersion is slow and uneven, it is rated as "poor"; if the dispersion is slow, there are agglomerates or particles during the dispersion process, and it can be dispersed evenly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com