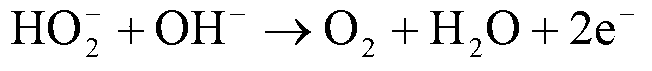Preparation method of carbon-free binder-free all-metal three-dimensional porous H2O2 electrooxidation catalytic electrode
A binder-free, three-dimensional porous technology, applied in the direction of battery electrodes, electrode carriers/current collectors, circuits, etc., can solve the problems of unsuitable electrodes, slow carbon oxidation, catalyst deactivation, etc., and achieve improved stability and preparation methods The effect of simplicity and a wide range of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
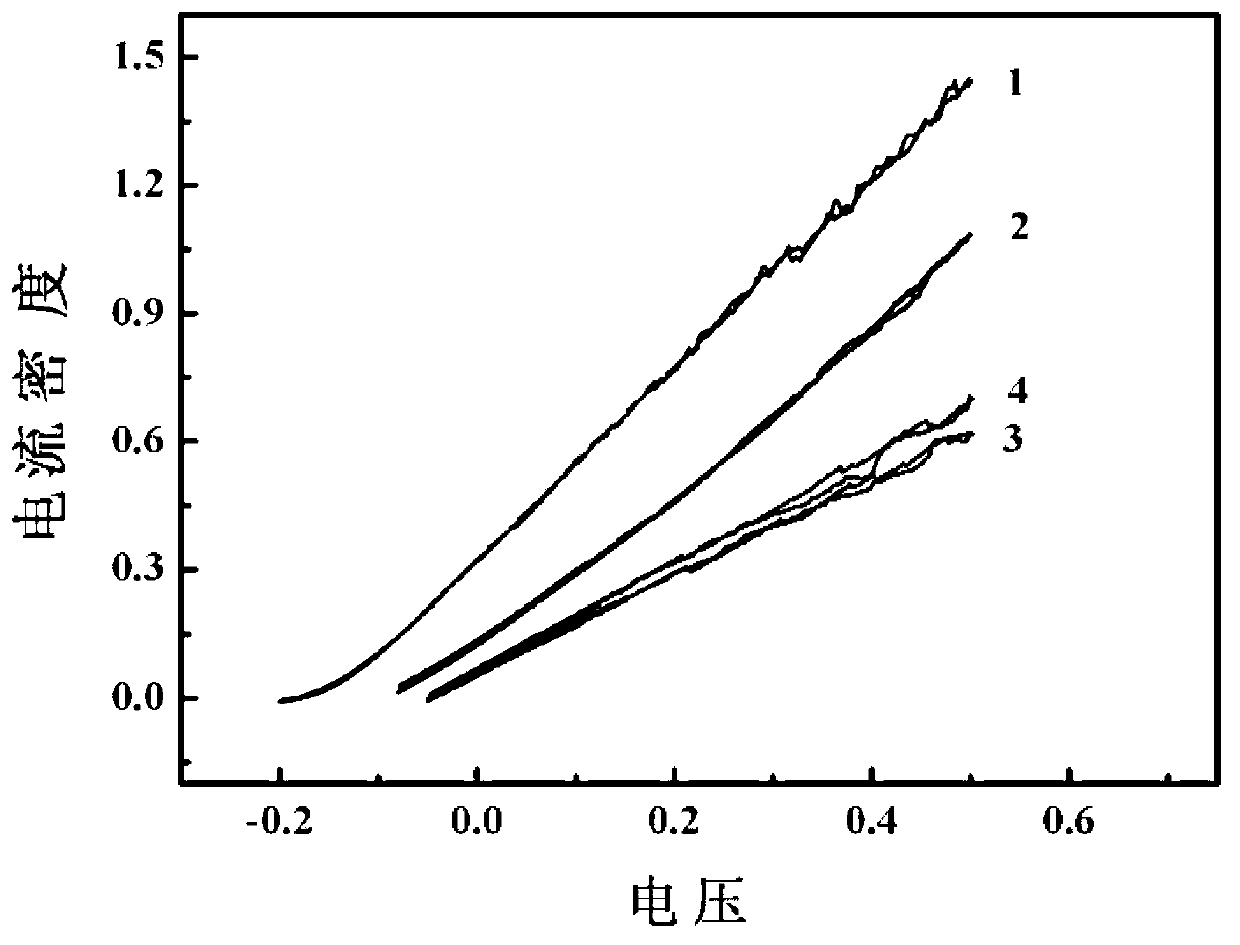
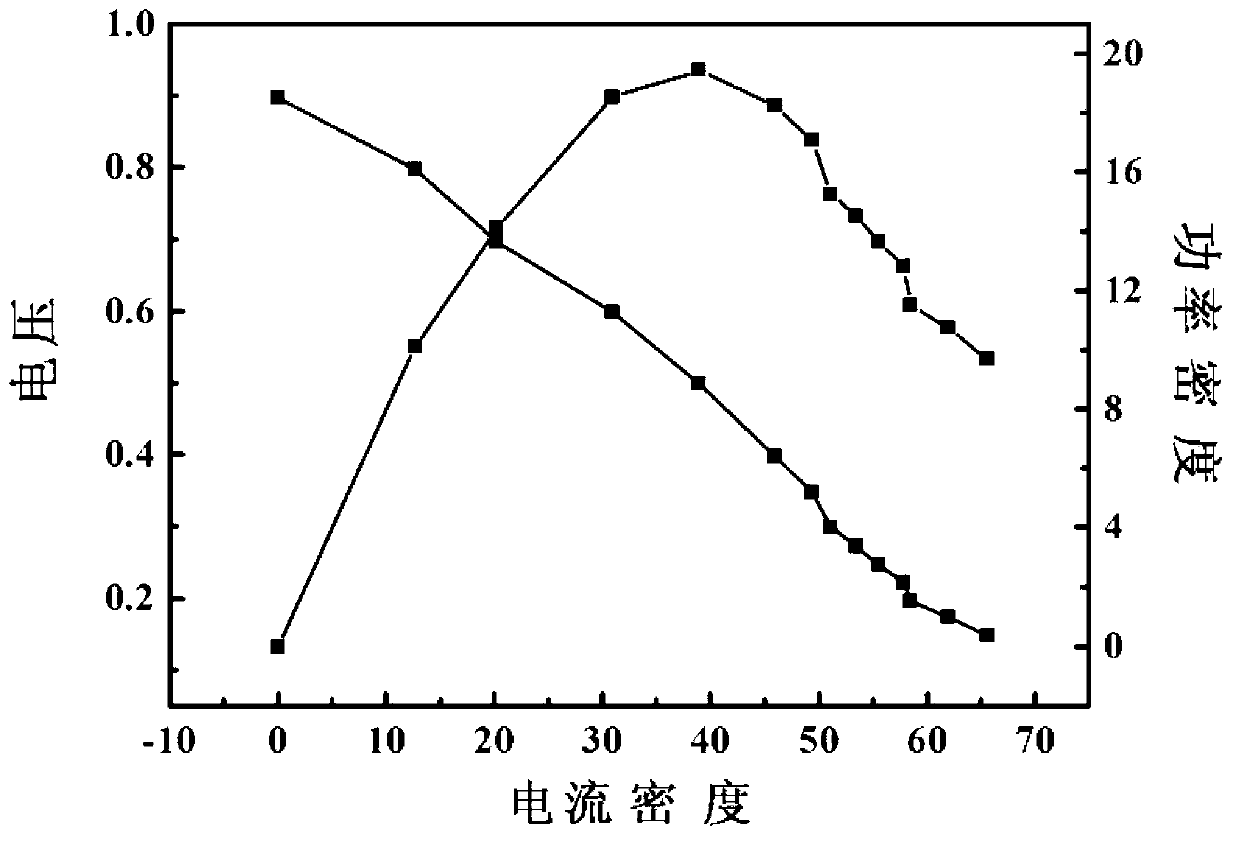
[0020] Use nickel foam as the matrix in 2.0mol L -1 NH 4 Cl+0.1mol L -1 NiCl 2 In deposition solution, constant current -2.0A cm -2 , deposited for 100 seconds to obtain Ni / Ni-foam electrodes. at 1.0mol L -1 h 2 o 2 +3.0mol L -1 The catalytic performance of hydrogen peroxide electrooxidation was tested in KOH solution, and the oxidation current on the Ni / Ni-foam electrode was 783mAcm at a potential of 0.2V. -2 , much larger than blank nickel foam (357mA cm -2 ) and the oxidation current on the noble metal Pd electrode (580mA cm -2 ). DPPFC composed of Ni / Ni-foam as anode and Pd / CFC as cathode, in which the anolyte is 4.0mol L -1 KOH+1.0mol L -1 h 2 o 2 , the catholyte is 2.0mol L -1 h 2 SO 4 +2.0mol L -1 h 2 o 2 , the flow rate is 10mL min –1 , The test temperature is 20°C. The open circuit voltage of the battery is as high as 0.9V, and the peak power reaches 19.4mW cm -2 .
Embodiment 2
[0022] Use nickel foam as the matrix in 0.05mol L -1 NH 4 Cl+0.1mol L -1 CTAB+0.4mol L -1 CoSO 4In the deposition solution, a constant voltage of -3V was deposited for 90 seconds to obtain a Co / Ni-foam electrode. at 1.0mol L -1 h 2 o 2 +3.0mol L -1 The electrooxidation performance of hydrogen peroxide was tested in KOH solution, and it was found that the oxidation current on the Co / Ni-foam electrode was 470mA cm at a potential of 0.2V. -2 . DPPFC composed of Co / Ni-foam as anode and Pd / CFC as cathode, in which the anolyte is 4.0mol L -1 KOH+1.0mol L -1 h 2 o 2 , the catholyte is 2.0mol L -1 h 2 SO 4 +2.0mol L -1 h 2 o 2 , the flow rate is 10mL min –1 , The test temperature is 20°C. The open circuit voltage of the battery is as high as 0.88V, and the peak power reaches 16.7mW cm -2 .
Embodiment 3
[0024] Stainless steel wire (Stainless steel wire) as the matrix in 1.5mol L -1 KSCN+0.1mol L -1 PVP+0.15mol L -1 Cu(NO 3 ) 2 In the deposition solution, the upper limit potential of pulse potential electrodeposition is 0.5V, the lower limit potential is -2.5V, the frequency is 10Hz, and the deposition is 200 seconds to obtain a Cu / SSW electrode. at 1.0mol L -1 h 2 o 2 +3.0mol L -1 The electrooxidation performance of hydrogen peroxide was tested in KOH solution, and it was found that the oxidation current on the Cu / SSW electrode was 300mA cm at a potential of 0.2V -2 . DPPFC composed of prepared Cu / SSW as anode and Pd / CFC as cathode, in which the anolyte is 4.0mol L -1 KOH+1.0mol L -1 h 2 o 2 , the catholyte is 2.0mol L -1 h 2 SO 4 +2.0mol L -1 h 2 o 2 , the flow rate is 10mL min –1 , The test temperature is 20°C. The open circuit voltage of the battery is as high as 0.85V, and the peak power reaches 15.6mW cm -2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com