Preparation method of paclitaxel or docetaxel octreotide conjugate
A technology for docetaxel and mono-docetaxel, which is applied in the field of drug synthesis and can solve the problems of unavailability of octreotide resin complexes, expensive catalysts and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
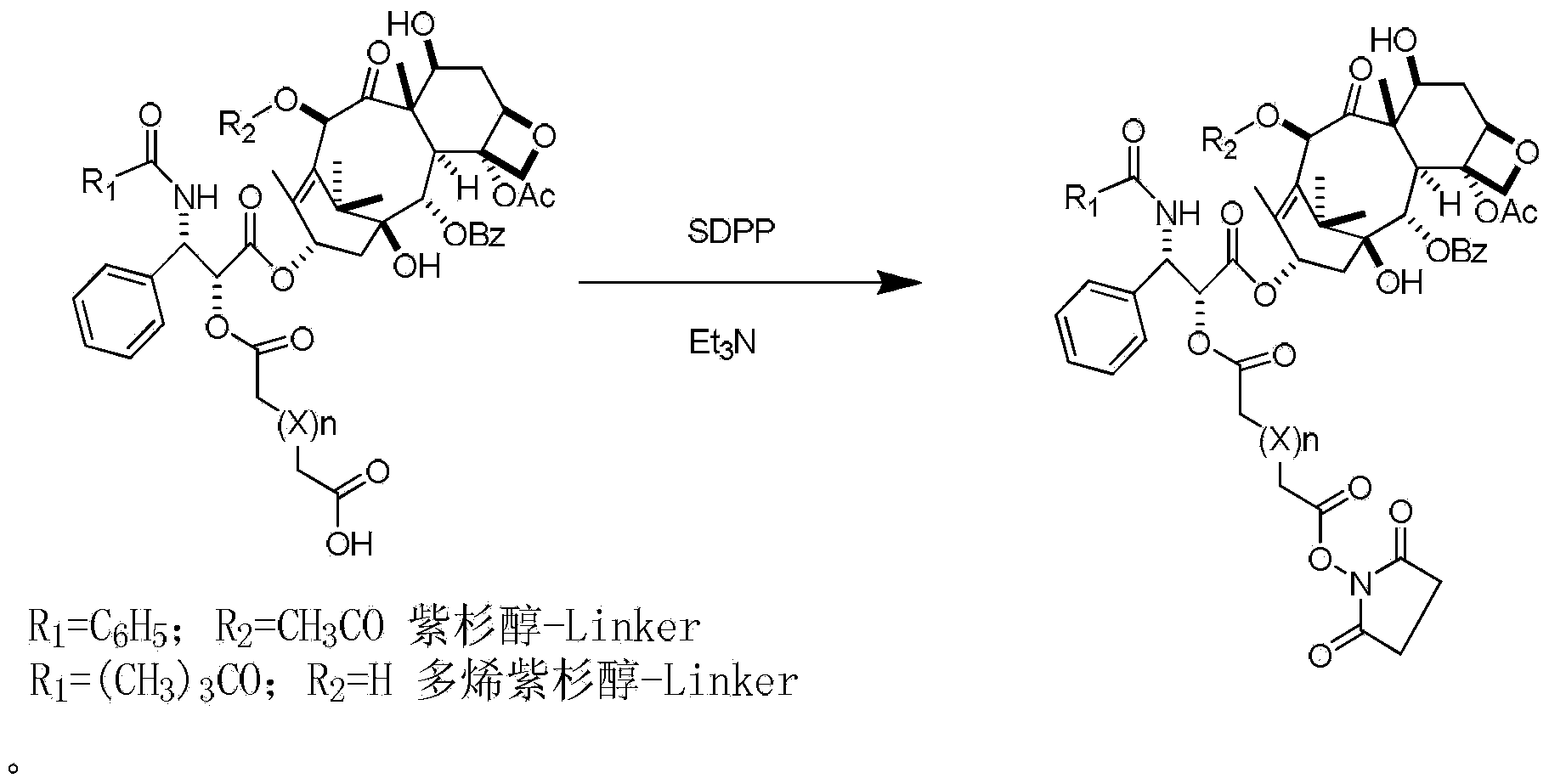
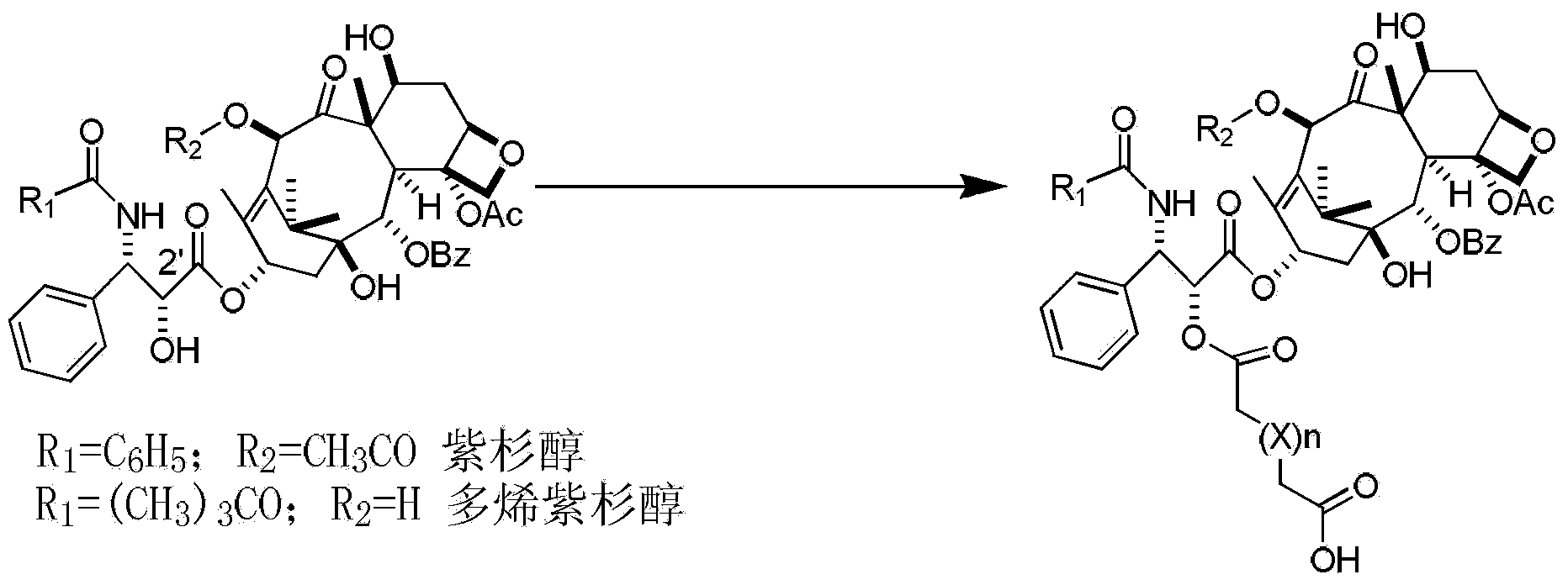
[0038] Vacuum-dry paclitaxel 200mg (0.234mmol) and succinic anhydride 300mg (3mmol) for 5 hours, dissolve in 5ml of dry pyridine, stir and react at 30°C for 24h, recover the solvent under reduced pressure to dryness, stir the residue with 10ml of ice water, filter The residue was dissolved in 10ml of acetone, 10ml of water was added dropwise under stirring, the solid was filtered out, and dried under reduced pressure to obtain paclitaxel succinate with a yield of 65%, a purity of 96%, ESI-MS (m / z): 976 [M+Na ] + ;992[M+K] + .
Embodiment 2
[0040]Docetaxel 200mg (0.248mmol) and diglycolic anhydride 348mg (3mmol) were vacuum-dried for 5 hours, dissolved in 5ml of dry pyridine, stirred and reacted at 30°C for 24h, the solvent was recovered under reduced pressure to dryness, and the residue was stirred with 10ml of ice water , the residue after filtration was dissolved in 10ml of acetone, and 10ml of water was added dropwise under stirring, the solid was precipitated by filtration, and dried under reduced pressure to obtain docetaxel diglycolate, with a yield of 63%, a purity of 93%, ESI-MS (m / z) : 946[M+Na] + .
Embodiment 3
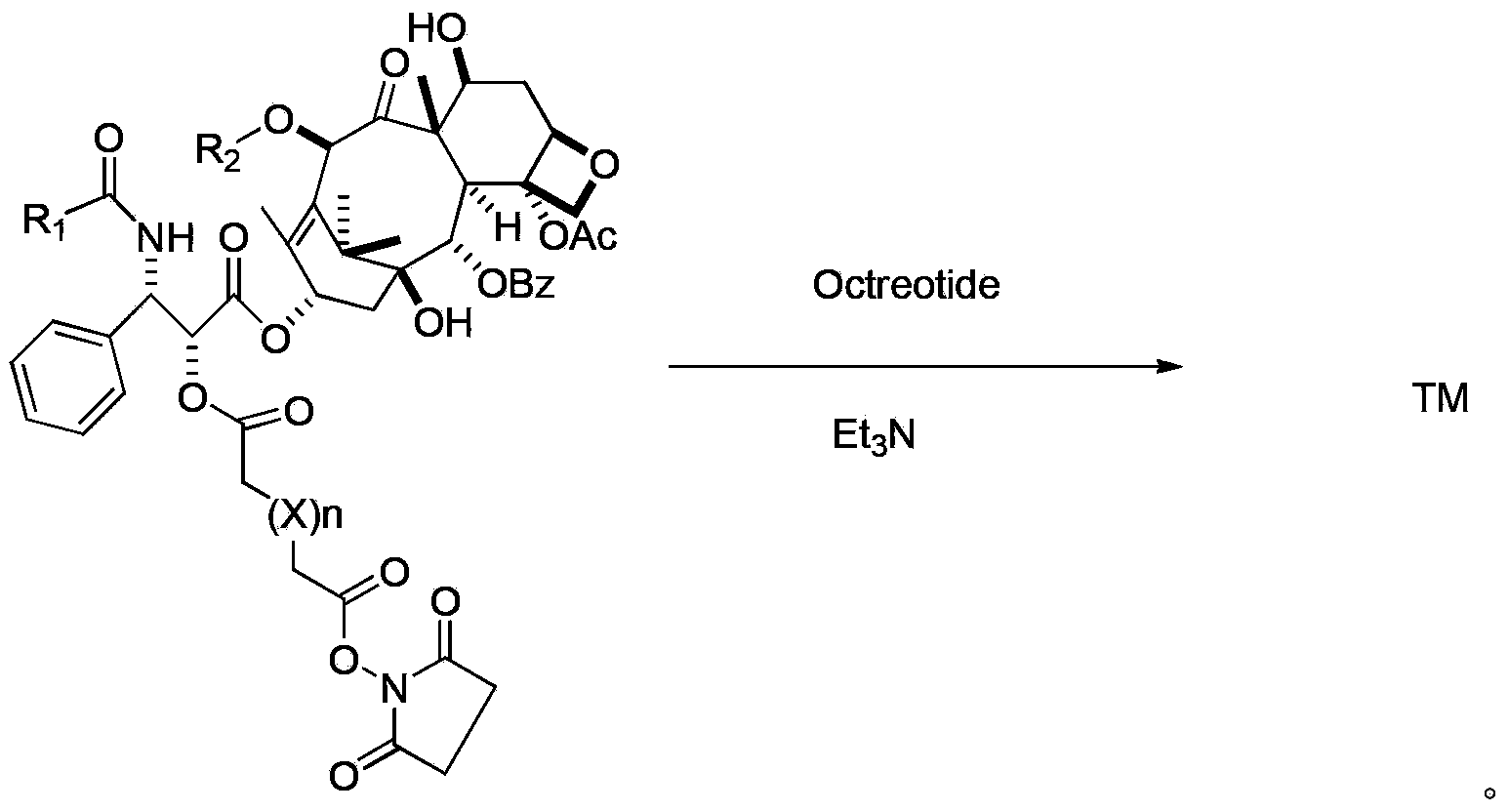
[0042] Dissolve 25 mg (0.025 mmol) of paclitaxel succinate, 30 mg (0.086 mmol) of SDPP and 30 mg (0.296 mmol) of triethylamine in 0.5 ml of anhydrous acetonitrile, stir at room temperature overnight, and after the reaction is detected by TLC, concentrate in vacuo, and the crude product The mixed solution was dissolved in ethyl acetate, washed with saturated brine, dried and recovered from the solvent to obtain the target product, ESI-MS (m / z): 1052.15 [M+H] + , directly used in the next reaction without further purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com