Octreotide acetate injection and preparation process thereof
A technology for the preparation of octreotide acetate, which is applied in the field of octreotide acetate injection and its preparation, can solve the problems of increased production process risk, poor stability, and accelerated growth rate, and achieve convenient product transportation and distribution, good safety, and mature technology Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
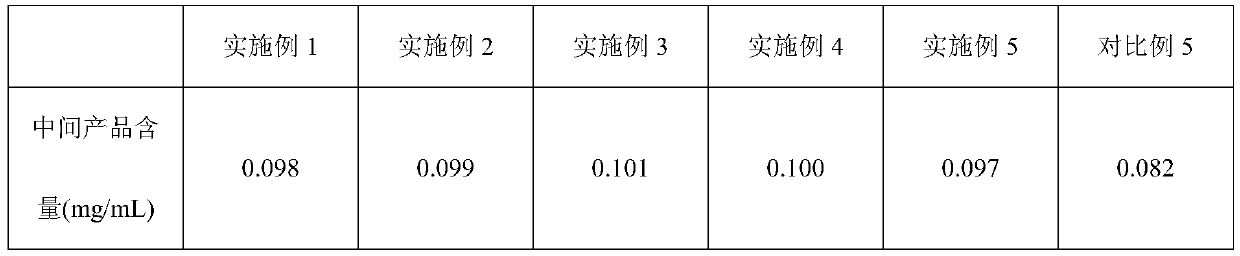
[0029] Octreotide acetate injection, based on 50,000 mL of the injection, the injection includes the following components: 5.0 g of octreotide acetate, 2250.0 g of mannitol, 169.3 g of lactic acid, an appropriate amount of sodium bicarbonate, and the balance is water for injection, Make 50,000 injections.
[0030] Preparation Process:
[0031] The solution preparation temperature is 20°C, the pH value is 4.0, the cellulose ester filter membrane is mixed, and the magnetic stirring method is used.
[0032] 1. Solution preparation
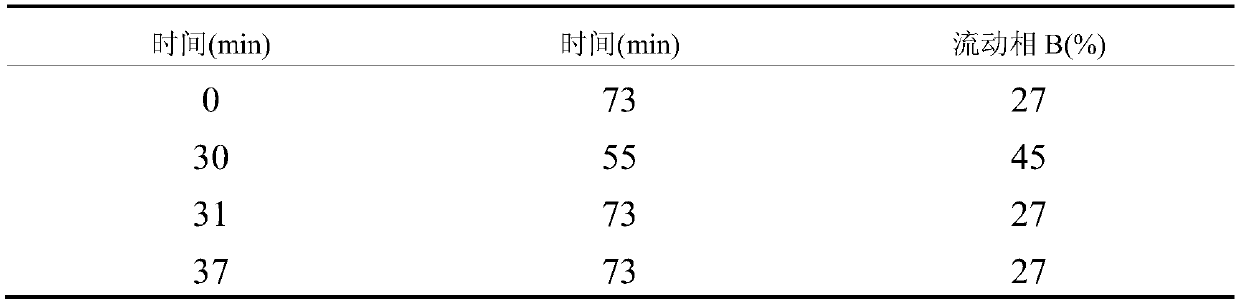
[0033] 1.1 Weigh the formula amount of mannitol into the magnetic stirring batching tank, add water for injection to 45,000 mL, the water temperature is 20-30 ° C, stir while adding to dissolve completely; add 169.3 g of lactic acid, stir for 5 minutes to mix evenly; Add 0.05% to the solution according to the mass volume ratio, and stir for 60 minutes; connect the sterilized cylindrical filter to the magnetic stirring batching tank, and decarbonize ...
Embodiment 2
[0039] Octreotide acetate injection, based on 50,000 mL of the injection, the injection includes the following components: 5.0 g of octreotide acetate, 2250.0 g of mannitol, 169.3 g of lactic acid, an appropriate amount of sodium bicarbonate, and the balance is water for injection, Make 50,000 injections.
[0040] Preparation Process:
[0041] The solution preparation temperature is 24°C, the pH value is 4.2, the cellulose ester filter membrane is mixed, and the magnetic stirring method is used.
[0042] 1. Solution preparation
[0043]1.1 Weigh the formula amount of mannitol into the magnetic stirring batching tank, add water for injection to 45,000 mL, the water temperature is 20-30 ° C, stir while adding to dissolve completely; add 169.3 g of lactic acid, stir for 5 minutes to mix evenly; Add 0.05% to the solution according to the mass volume ratio, and stir for 30 minutes; connect the sterilized cylindrical filter to the magnetic stirring batching tank, and decarbonize t...
Embodiment 3
[0049] Octreotide acetate injection, based on 50,000 mL of the injection, the injection includes the following components: 5.0 g of octreotide acetate, 2250.0 g of mannitol, 169.3 g of lactic acid, an appropriate amount of sodium bicarbonate, and the balance is water for injection, Make 50,000 injections.
[0050] Preparation Process:
[0051] The solution preparation temperature is 22°C, the pH value is 4.1, mixed with cellulose ester filter membrane, and magnetically stirred.
[0052] 1. Solution preparation
[0053] 1.1 Weigh the formula amount of mannitol into the magnetic stirring batching tank, add water for injection to 45,000 mL, the water temperature is 20-30 ° C, stir while adding to dissolve completely; add 169.3 g of lactic acid, stir for 5 minutes to mix evenly; Add 0.05% to the solution according to the mass volume ratio, and stir for 60 minutes; connect the sterilized cylindrical filter to the magnetic stirring batching tank, and decarbonize the solution throu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com