Method for producing aromatic hydrocarbon and ethylene by taking naphtha as raw material
A technology for naphtha and aromatics, which is applied in naphtha processing, chemical instruments and methods, and the petroleum industry, and can solve problems such as increasing demand for naphtha and low normal paraffin content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
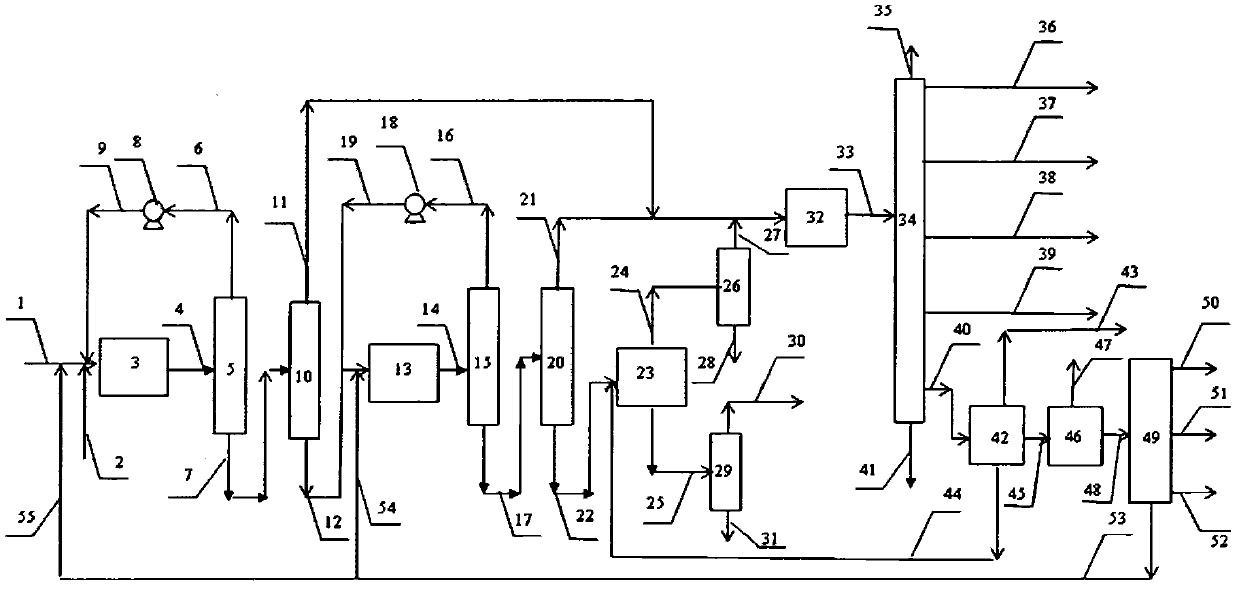
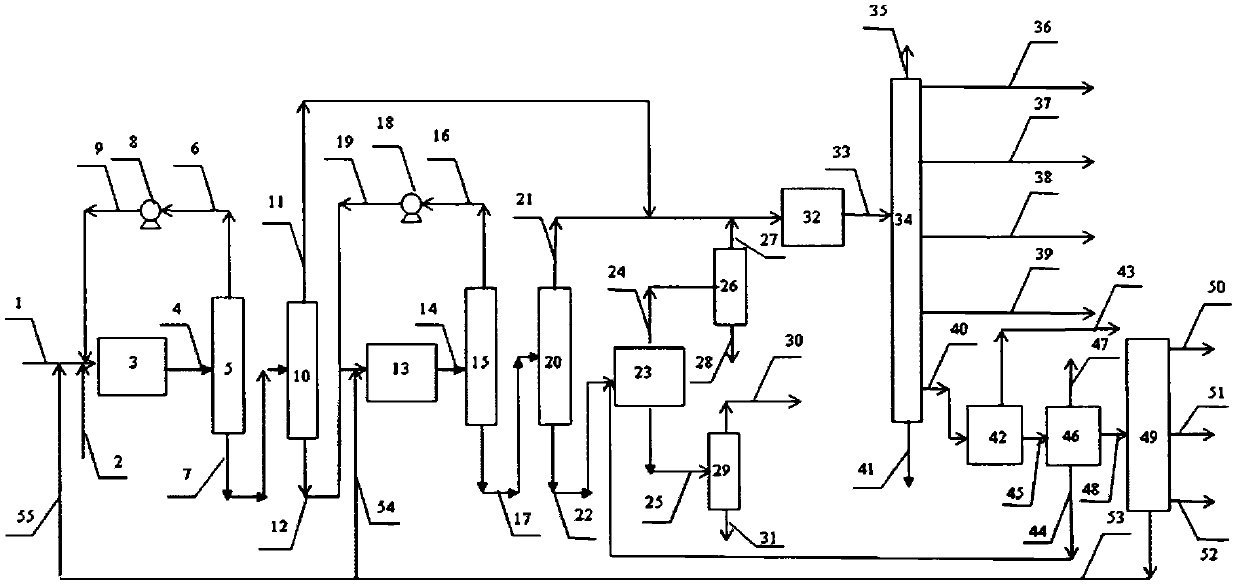
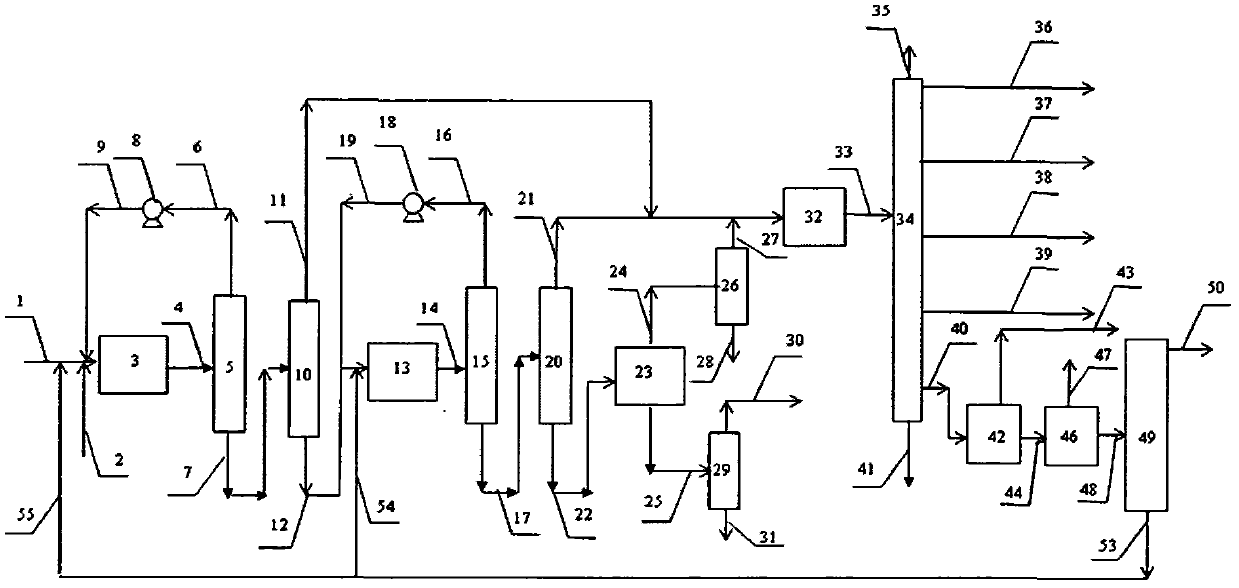
Image
Examples
example 1
[0086] In this example, naphtha is hydrorefined.
[0087]In a 20 milliliter fixed bed continuous flow reactor, fill 20 milliliters of hydrorefining catalyst A (RS-1 catalyst, produced by Sinopec Catalyst Changling Branch Company), which contains 0.03 mass % CoO, 2.0 mass % NiO, 19.0 mass% WO 3 , 0.7% by mass of F and 78.27% by mass of Al 2 o 3 .
[0088] The naphtha with the composition and properties listed in Table 1 was heated at 290°C, the hydrogen partial pressure was 1.6MPa, the hydrogen / hydrocarbon volume ratio was 200:1, and the feed volume space velocity was 8.0h -1 Under certain conditions, it is passed into the above-mentioned reactor filled with catalyst A for hydrogenation refining, and the reaction product enters the water cooler and is separated into two phases of gas and liquid, which are measured separately and analyzed for composition. The composition and properties of the naphtha obtained after refining are shown in Table 2.
[0089] Table 1
[0090] ...
example 2~3
[0095] The following examples carry out catalytic reforming according to the method of the present invention.
[0096] Using PtSn / γ-Al 2 o 3 Catalyst B (GCR-100A, produced by Hunan Jianchang Petrochemical Co., Ltd.), which contains 0.35% by mass of Pt, 0.30% by mass of Sn, 1.0% by mass of Cl, and the balance is γ-Al 2 o 3 .
[0097] In 100 milliliters of fixed-bed continuous flow reactors, fill 50 milliliters of catalyst B, be the catalytic reforming raw material with the refined naphtha listed in table 2, be 500 ℃ at reaction mass inlet temperature, reaction pressure be 0.34MPa, hydrogen / The hydrocarbon molar ratio is 6.7, and the feed volume space velocity is 20.0h -1 、8.0h -1 The reforming reaction is carried out under certain conditions, and the reforming reaction product is subjected to gas-liquid separation and rectification to obtain reformed liquefied gas and C 5 + Reforming produces oil, and the reaction results are shown in Table 3.
example 4
[0099] Using PtRe / γ-Al 2 o 3 Catalyst C (CB-60 catalyst, produced by Sinopec Catalyst Changling Branch), which contains 0.26% by mass of Pt, 0.26% by mass of Re, 1.0% by mass of Cl, and the balance is γ-Al 2 o 3 .
[0100] In a 100 milliliter fixed-bed continuous flow reactor, 50 milliliters of catalyst C was filled, and catalyst C was presulfurized by adding 0.1 mass % hydrogen sulfide in a hydrogen stream at 425 ° C before use, so that the sulfur content of the catalyst was 0.06 mass %. The refined naphtha listed in Table 2 is used as the raw material for catalytic reforming, when the reaction raw material inlet temperature is 475°C, the reaction pressure is 1.4MPa, the hydrogen / hydrocarbon molar ratio is 6.7, and the feed volume space velocity is 20.0h -1 The reforming reaction is carried out under certain conditions, and the reforming reaction product is subjected to gas-liquid separation and rectification to obtain reformed liquefied gas and C 5 + Reforming produces ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com