Special-shaped dual functional coupling agent-assisted PEG (polyethylene glycol) modifying method for protein
A bifunctional linker and modification method technology, applied in the field of protein chemistry, can solve the problems of unfavorable production control stability and high yield of PEG multi-modification, and achieve the effect of separation and purification and high yield of single modification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
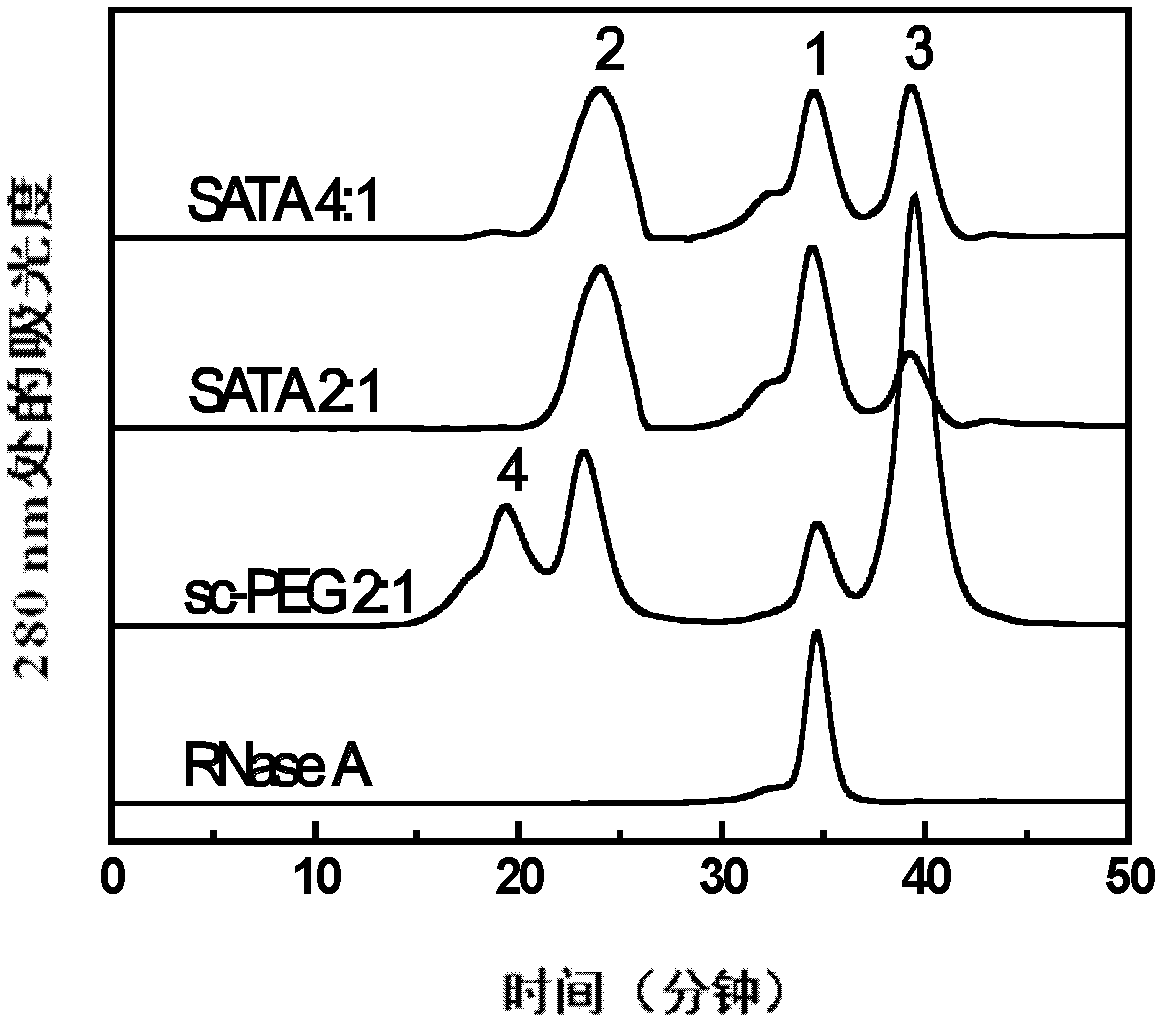
[0029] Embodiment 1SATA assists the PEG modification of RNaseA
[0030] experimental method
[0031] RNaseA was dissolved in 20 mM phosphate buffer solution (pH 7.4). SATA was first dissolved in N,N-dimethylformamide, and then a certain amount of 20mM phosphate buffer (pH 7.4) was added. According to the molar ratio of RNase A and SATA being 1:2 and 1:4, a certain amount of SATA solution was added to the RNaseA solution respectively, shaken and mixed, and left to stand at 4° C. for 3 hours to react.
[0032] Hydroxylamine was dissolved in 20 mM phosphate buffer (pH 7.4), and a certain amount of hydroxylamine solution was added to the mixture of RNaseA and SATA according to the molar ratio of RNaseA and hydroxylamine at a ratio of 1:20. After shaking, let stand at 4°C for 2 hours to react.
[0033] The reaction solution was transferred to an ultrafiltration tube with a molecular weight cut-off of 5kDa, and the centrifugation speed was set to 5000g to remove unreacted SATA, h...
Embodiment 2
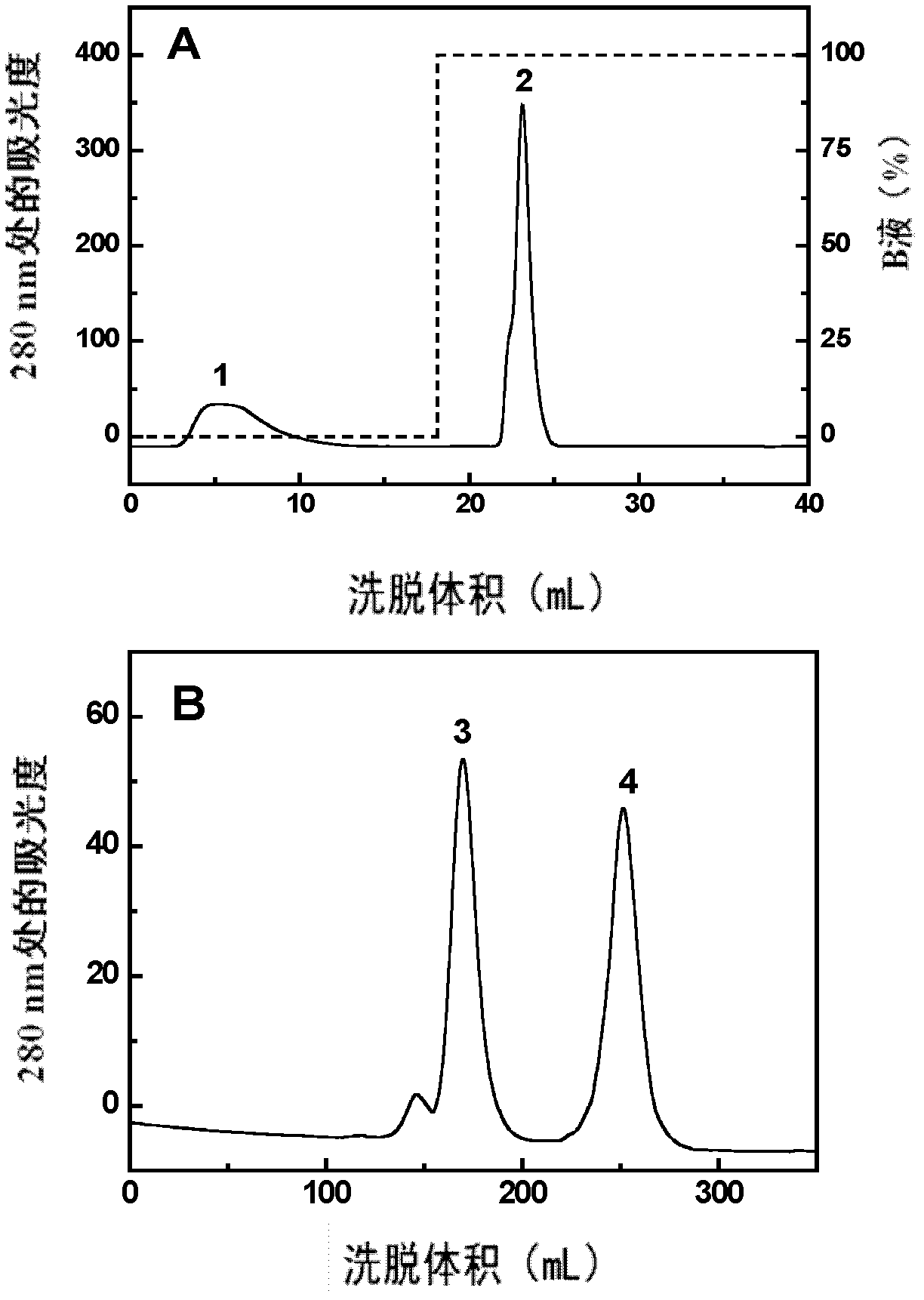
[0038] The separation, purification and identification of embodiment 2PEG single modification product
[0039] experimental method
[0040] By means of dialysis, the above sample solution was replaced into 50 mM acetic acid-sodium acetate buffer solution (pH5.0, solution A). Using SP Sepharose High Performance cation exchange chromatography, the unreacted PEG in the sample was eluted with liquid A after loading the sample, and then 100% pH 5.0 was used, and 0.5M sodium chloride was added to the acetic acid-sodium acetate buffer solution ( Solution B) Elution of protein components. The eluted protein fractions were collected.
[0041] The PEG single-modified product of RNase A was separated and purified with a preparative chromatographic column Superdex 200 (2.6cm×70cm). The buffer system used was PBS buffer (pH 7.4), and the product was collected and concentrated.
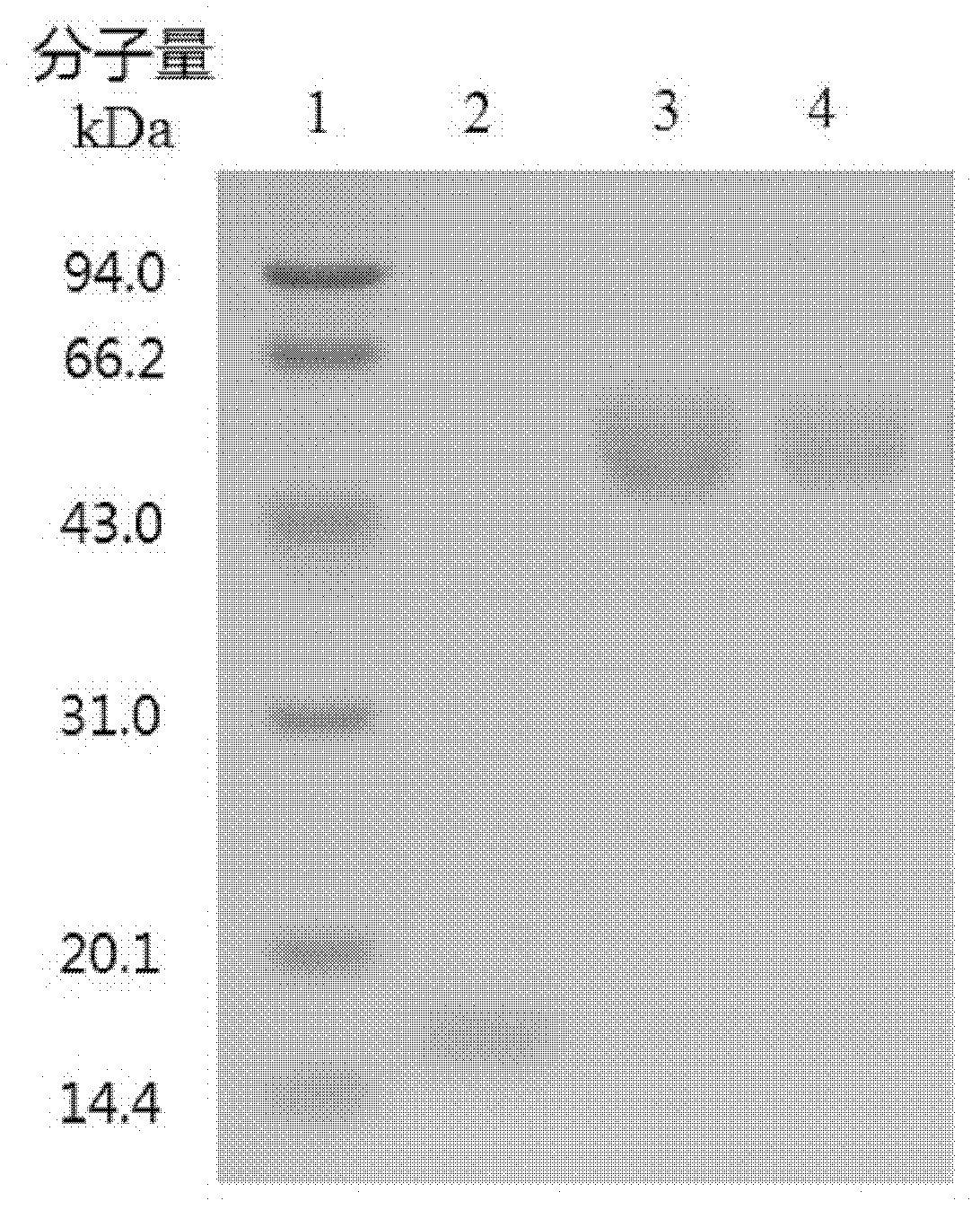
[0042] The purified samples were identified by reducing SDS-PAGE. Using PolyA-PolyU sodium salt as the subs...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com