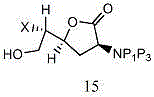
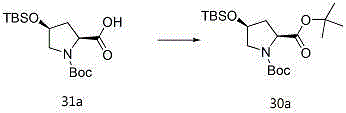
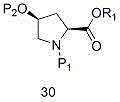
Entecavir intermediate and preparation method thereof
A stereo configuration and compound technology, applied in the field of hepatitis B drug intermediates and its preparation, can solve the problems of low efficiency of chiral groups, low purity and yield, selection of guanine ring-opening position and poor chiral selectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0133] Example 1 (synthesis of compound 13a, R1=Ph)
[0134]
[0135] Under nitrogen protection, add benzaldehyde (200g, 1887mmol, 8.3eq) into a 3000mL reaction flask, add anhydrous zinc chloride (50g, 366.8mmol, 1.62eq) with electric stirring, add N-acetyl-D-glucosamine 14a ( 50g, 226mmol, 1eq), after reacting at 20-40°C for 18 hours, controlled by HPLC, 14a is less than 1%, stop the reaction, add 740g of ethyl acetate to make slurry for 2 hours, filter, wash the filter cake with a large amount of ethyl acetate, blow After drying for 6 hours, 60 g of white solid 14a was obtained, with a yield of 86%.
example 2
[0136] Example 2 (synthesis of compound 13a, R1=Ph)
[0137] Under nitrogen protection, add benzaldehyde (200g, 1887mmol, 8.3eq) into a 3000mL reaction flask, add anhydrous p-toluenesulfonic acid monohydrate (0.45g, 2.26mmol, 0.01eq) with electric stirring, add N-acetyl-D -Glucosamine 14a (50g, 226mmol, 1eq), reacted at 20-40°C for 18 hours, controlled by HPLC, 14a was less than 1%, stopped the reaction, added 740g ethyl acetate, beat at 65°C for 6 hours, filtered, and the filter cake was large Washed with ethyl acetate and air-dried for 6 hours to obtain 55 g of 13a as a white solid, with a yield of 78.6%.
example 3
[0138] Example 3 (synthesis of compound 12a, R1=Ph, P2=P4=Ac)
[0139]
[0140] Add 13a (50g, 161.7mmol, 1eq) and pyridine (680g, 8597mmol, 53eq) into a 1L reaction flask. After addition, add acetic anhydride (320g, 3134.4mol, 19.4eq) dropwise, control the temperature at 20-30°C, drop After the addition, heat up to 40-45°C and react for 10 hours. Control 13a in HPLC to less than 1%, control the temperature below 60°C to distill the solvent pyridine under reduced pressure, steam until the system is viscous, stop the distillation, cool down to 15-25°C, and add 340g Methyl tertiary ether was stirred for 5 hours, filtered, and air-dried to obtain 55.9 g of white solid 12a, with a yield of 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com