Method for synthesizing ivermectin
A technology of ivermectin and avermectin, applied in the field of synthesizing ivermectin, can solve problems such as expensive rhodium catalyst
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] The present invention will be further described in detail below in conjunction with the embodiments.
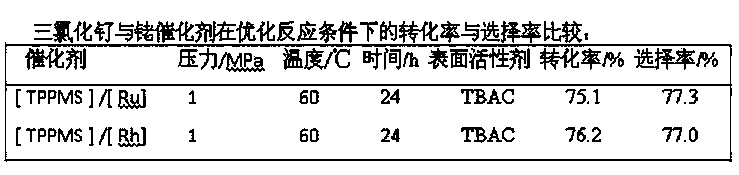
[0020] The invention provides a method for synthesizing ivermectin. The method is catalyzed by ruthenium trichloride and monosulfonated triphenylphosphine sodium salt, avermectin and hydrogen are hydrogenated to obtain ivermectin .
[0021] Compared with rhodium, the price of ruthenium is only about 1 / 10 of that of rhodium, and the use of the catalyst of the invention can reduce the synthesis cost of ivermectin by about 30%.
[0022] Preferably, the molar ratio of the monosulfonated triphenylphosphine sodium salt to ruthenium trichloride (3-5):1.
[0023] Preferably, the mass ratio of ruthenium trichloride to abamectin is 1: (10-20).
[0024] Preferably, the hydrogenation reaction is carried out in a water-organic solvent two-phase system.
[0025] The water-organic solvent two-phase system refers to the mixture of water and organic solvent to form a liquid-liquid two-phase mixe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com