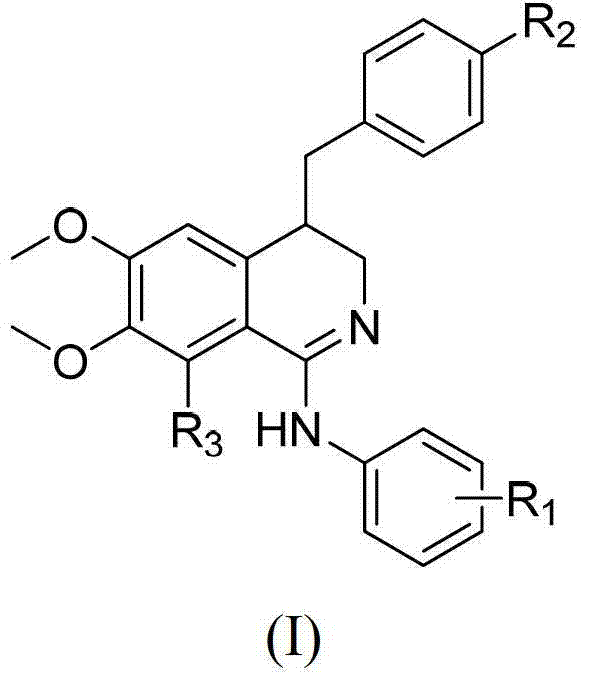
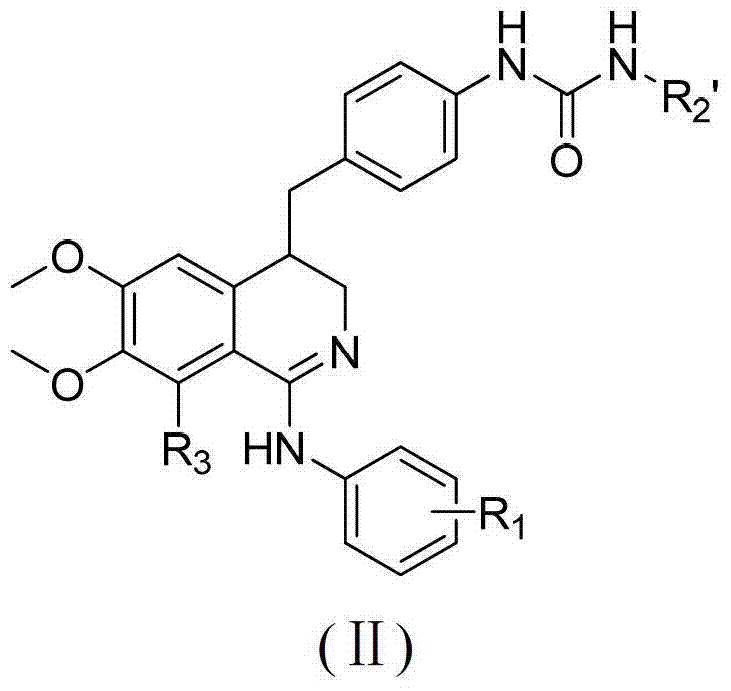
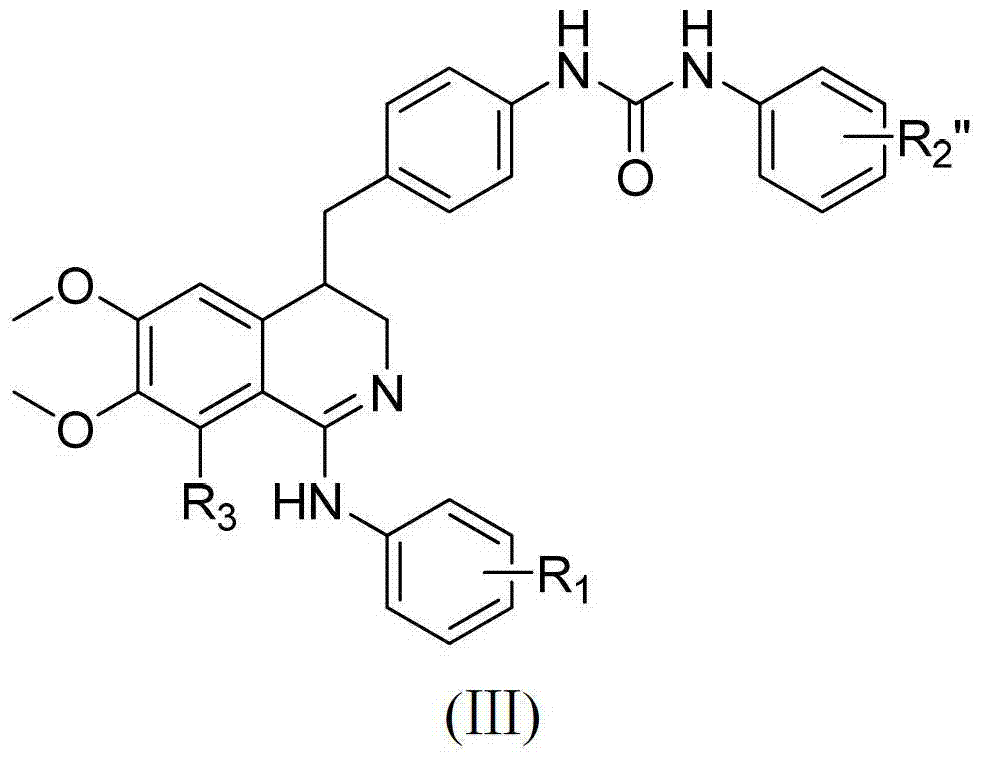
3, 4-dihydroisoquinoline antitumor compounds as well as preparation method and application thereof
A technology of dihydroisoquinoline and dimethoxyisoquinoline, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1: 4-(4-nitrobenzyl)-N-(3-chlorophenyl)-3,4-dihydro-6,7-dimethoxyisoquinolin-1-amine (H- 1) Preparation
[0073] A. Preparation of (Z) 2-(3,4-dimethoxyphenyl)-3-(4-nitrophenyl)acrylonitrile
[0074] Weigh 1.77g of 3,4-dimethoxyphenylacetonitrile (0.010mol) into a 250ml round bottom flask, then add 1.81g of p-nitrophenylacetonitrile (0.012mol) and 40ml of ethanol, there is a large amount of insoluble matter, then add 0.40g (0.010mol) sodium hydroxide, stirred under ice bath, completely dissolved after 1min. After 30 min, a large amount of yellow powder was precipitated, the reaction was stopped, the reaction solution was filtered, the solid was washed with ethanol three times, and then crystallized with ethyl acetate as a solvent to obtain 2.73 g of yellow crystals with a yield of 88.1%. The melting point is 134°C to 135°C. 1 H NMR (300MHz, CDCl 3 )δ8.46–8.26(m,2H),8.16–7.97(m,2H),7.48(s,1H),7.31(m,1H),7.17(d,J=2.3Hz,1H),6.95(d ,J=8.5Hz,1H),3.98(s,3H),3.96(s...
Embodiment 2
[0084] Embodiment 2: the preparation of 4-(4-aminobenzyl)-N-(3-chlorophenyl)-3,4-dihydro-6,7-dimethoxyisoquinolin-1-amine (H -2) Preparation
[0085]In a 100ml round bottom flask, add 40ml of methanol, 1.10g (0.0024mol) of compound H-1, stir and dissolve at room temperature, then add 3ml of 85% hydrazine hydrate (0.045mol) and 0.2g of Raney nickel, and stir for 0.5h at room temperature. After the reaction, filter with a sand core funnel, react the filter residue with dilute acid until there is no air bubbles, put it into the waste liquid bucket, evaporate the filtrate to dryness under reduced pressure, and obtain a white solid H-21.01g, the yield is 98.4%, and it can be put into it without purification Next reaction.
[0086] The preparation methods of compounds H-13 and H-22 are the same as in Example 2.
Embodiment 3
[0087] Example 3: 1-(4-((1-(3-chlorophenylamino)-3,4-dihydro-6,7-dimethoxyisoquinolin-4-yl)methyl)phenyl )-3-(4-(trifluoromethyl)phenyl)urea (H-9) preparation
[0088] In a 50ml round bottom flask, add 10ml THF, 0.20g (0.00047mol) compound H-2, stir and dissolve at room temperature, then add 0.09g (0.00047mol) 4-trifluoromethylphenylisocyanate, and continue stirring for 24h. After the reaction, evaporate under reduced pressure until a small amount of solvent remains (less than 1ml), and separate by reverse-phase medium-pressure column chromatography with methanol / water as the mobile phase. After separation, 0.15 g of white solid H-9 is obtained, with a yield of 51.7%.
[0089] The preparation methods of compounds H-3~H-8, H-10~H-11, H-14~H-20 and H-23 are the same as in Example 3.
[0090] The chemical structure, physicochemical constants, 1 H NMR and ESI-MS data are shown in Table 1 and Table 2.
[0091] Table 1. Compound structure, physical and chemical constants, molecul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com