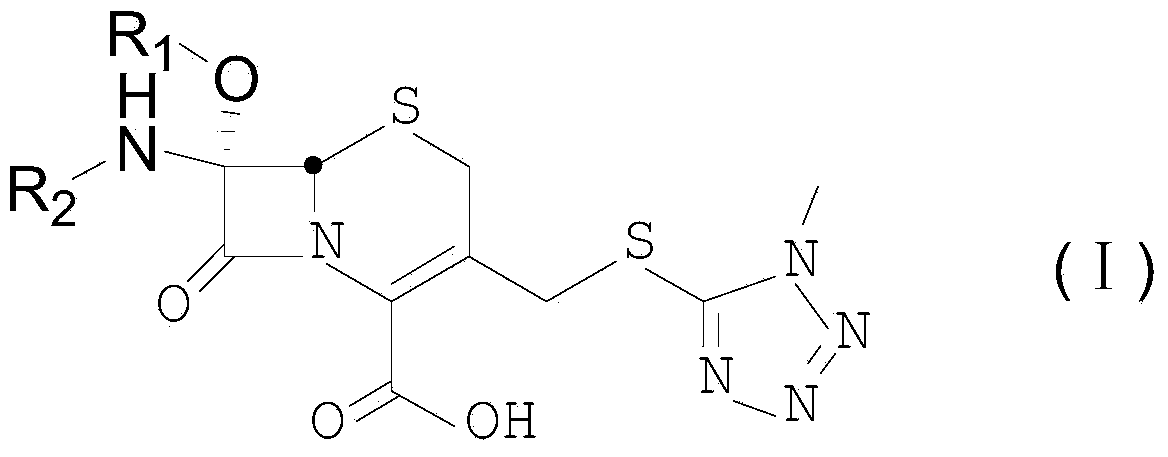
Cephalosporin compound and preparation method thereof
A technology for compounds and cephalosporins, applied in the field of cephalosporins and their preparation, can solve problems such as restricting the development of antibiotics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
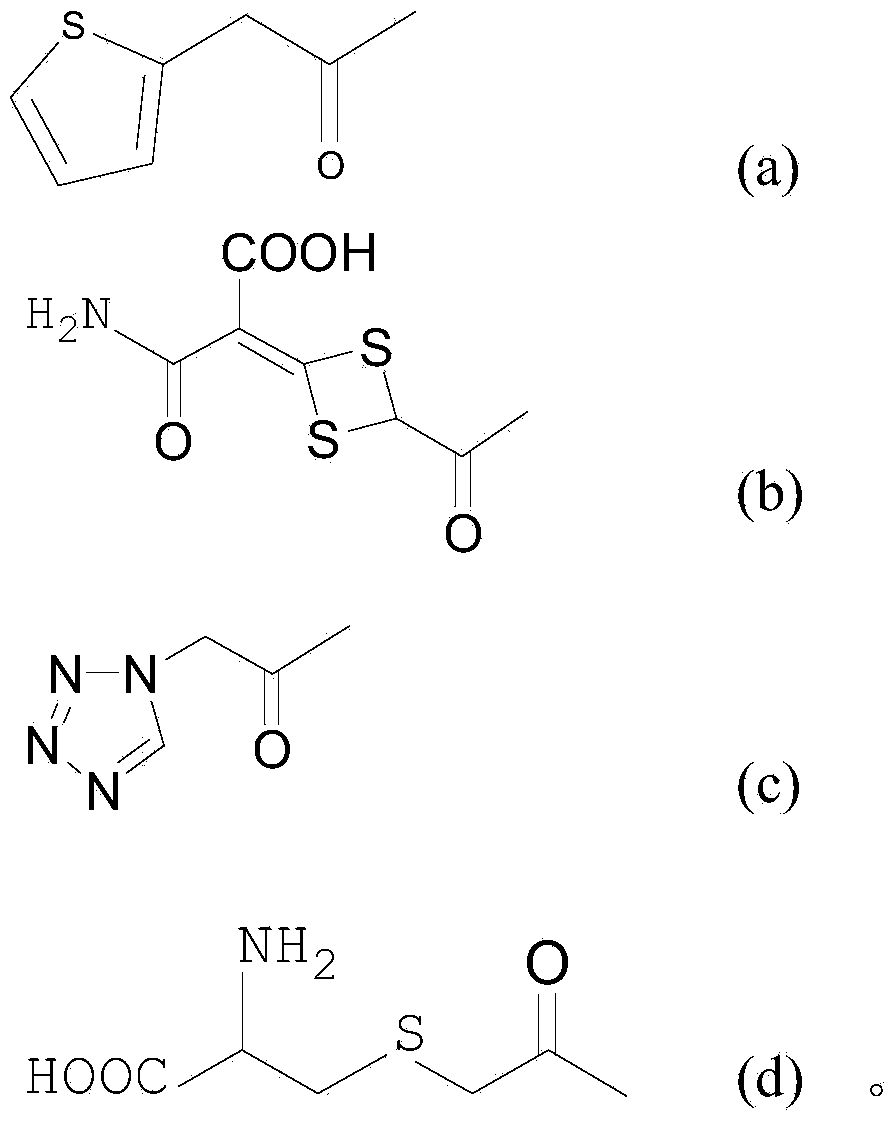
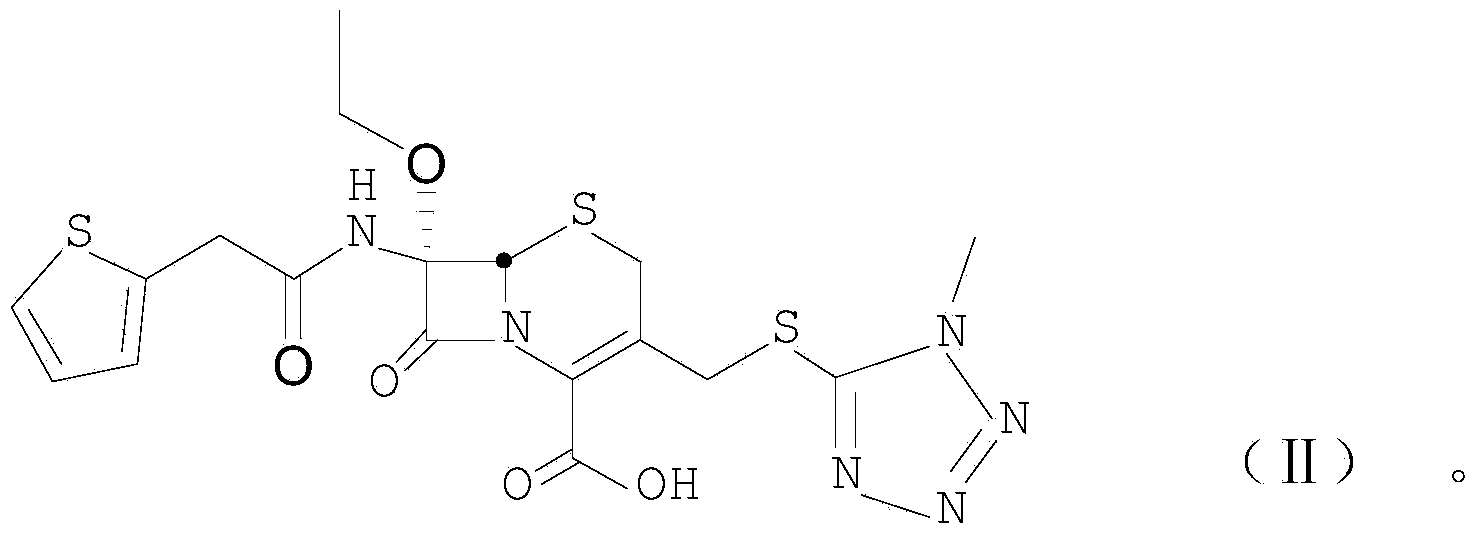
[0033] Example 1: Synthesis of product 7α-ethoxy-7β-(2-thienylacetamido)-3-[(1-methyl-1H-tetrazol-5-yl)thiomethyl]cephalosporanic acid
[0034]
[0035] Step (1): 0.25 g of benzhydryl 7α-ethoxy-7β-amino-3-[(1-methyl-1H-tetrazol-5-yl)thiomethyl]cephem-4-carboxylate Dissolve in 10ml of ethyl acetate, cool down to -5°C, add 0.20g of DMAP (N,N-lutidine), dropwise add 5ml of dichloromethane containing 0.20g of thiopheneacetyl chloride, and monitor the reaction by TLC (benzene: petroleum ether=1:2). After about 6 hours of reaction, wash with water, and then wash the organic layer with 10% sodium bisulfate solution, then wash with water, and spin dry the organic layer.
[0036] Step (2): 7α-ethoxy-7β-(2-thienylacetamido)-3-[(1-methyl-1H-tetrazol-5-yl)thiomethyl]cephalosporanic acid
[0037] Step (3): Dissolve the product from the previous step with 20ml of dichloromethane, add 0.2ml of anisole, cool down to -5°C, flow HCl gas, and monitor the reaction by TLC (benzene:petroleum e...
Embodiment 2
[0038] Example 2: Product 7α-ethoxy-7β-[[4-(1-amino-3-hydroxyl-1,3-dioxopropane-2-yl subunit)-1,3-dithioheterocycle Synthesis of butane-2-formyl]amino]-3-[(1-methyl-1H-tetrazol-5-yl)thiomethyl]cephalosporanic acid
[0039]
[0040] Step (1): 0.25 g of benzhydryl 7α-ethoxy-7β-amino-3-[(1-methyl-1H-tetrazol-5-yl)thiomethyl]cephem-4-carboxylate Dissolve in 10ml of dichloromethane, cool down to -5°C, add 0.20g of N,N-xylaniline, dropwise add 5ml of dichloromethane containing 0.20g of bromoacetyl bromide, and monitor the reaction by TLC (benzene:petroleum ether=1 :2). After about 2 hours of reaction, wash with water, and then wash the organic layer with 10% sodium bisulfate solution, then wash with water, and spin dry the organic layer.
[0041] Step (2): Dissolve the product from the previous step with 20ml of dichloromethane, add 0.2ml of anisole, cool down to -5°C, add 0.35ml of trifluoroacetic acid dropwise, and monitor the reaction by TLC (benzene:petroleum ether=1:2) , ...
Embodiment 3
[0043] Example 3: 7α-ethoxy-7β-[(S)-2-[(2-amino-2-carboxyethyl)thio]acetamido]-3-[(1-methyl-1H-tetrazole -5-base) thiomethyl] cephalosporanic acid synthesis,
[0044]
[0045] Step (1): Dissolve 0.25 g of benzhydryl 7α-ethoxy-7βamino-3-[(1-methyl-1H-tetrazol-5-yl)thiomethyl]cephem-4-carboxylate In 10ml of dichloromethane, lower the temperature to -5°C, add 0.20g of N,N-xylidine, dropwise add 5ml of dichloromethane containing 0.20g of bromoacetyl bromide, and monitor the reaction by TLC (benzene:petroleum ether=1: 2). After about 2 hours of reaction, wash with water, and then wash the organic layer with 10% sodium bisulfate solution, then wash with water, and spin dry the organic layer.
[0046] Step (2): Dissolve the product from the previous step with 20ml of dichloromethane, add 0.2ml of anisole, cool down to -5°C, add 0.35ml of trifluoroacetic acid dropwise, and monitor the reaction by TLC (benzene:petroleum ether=1:2) , After about 3 hours of reaction, spin dry direc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com