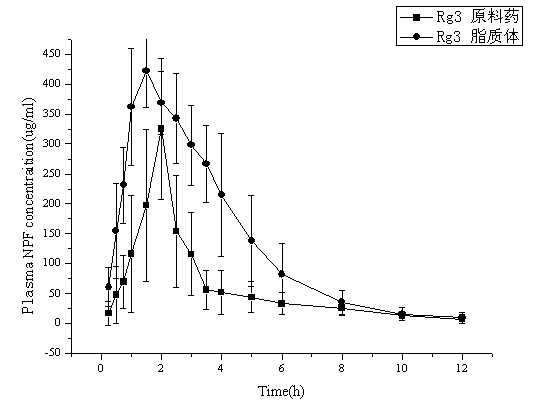
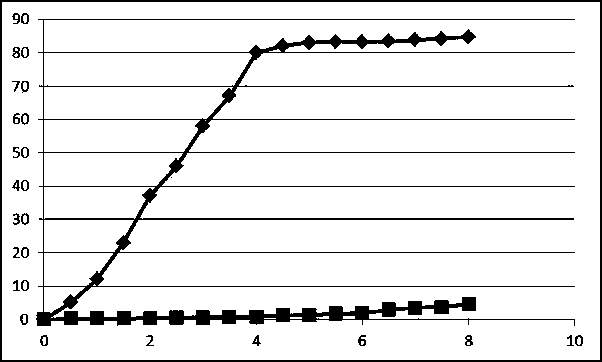
Ginsenoside Rg3 liposome and preparation method thereof
A technology of ginsenosides and liposomes, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]Weigh 0.5g ginsenoside Rg3, 7.5g egg yolk lecithin, and dissolve 1.25g cholesterol in 50ml n-butanol, place the solution in a pear-shaped bottle, evaporate under reduced pressure above the rotary evaporation to remove n-butanol, and make the phospholipids in the bottle A uniform film was formed on the wall, and the organic solvent was completely volatilized and removed by vacuum drying for 24 hours. 10ml of 10mM pH6.5 phosphate buffer solution was added to fully hydrate at the phospholipid phase transition temperature to obtain a ginsenoside Rg3 liposome suspension. The obtained liposome suspension was homogenized for 3 cycles through a high-pressure homogenizer, and then granulated through a 100nm polycarbonate membrane under high pressure through an extruder, and the encapsulation efficiency of the finally gained liposomes was 87.3%, the average particle size is 131nm.
Embodiment 2
[0046] Weigh 0.5g ginsenoside Rg3, 5g soybean lecithin, and dissolve 1.25g cholesterol in 50ml ethanol, and slowly inject the phospholipid ethanol solution into 20mL of 20mM pH6.5 phosphate buffer solution shaken in a constant temperature water bath at 50 degrees Celsius with a micro-syringe to obtain Ginsenoside Rg3 liposome suspension, the obtained liposome suspension is homogenized through a high-pressure homogenizer for 3 cycles, and then granulated through a 100nm polycarbonate membrane under high pressure by an extruder, An ultrafiltration membrane with a molecular weight cut-off of 5000 was selected, and ethanol was removed by ultrafiltration and concentration. The encapsulation efficiency of the finally obtained liposome was 81.2%, and the average particle size was 136nm.
Embodiment 3
[0048] Weigh 0.5g ginsenoside Rg3, 7.5g egg yolk lecithin, and dissolve 1.25g cholesterol in 50ml ethanol, slowly inject the phospholipid ethanol solution into 20mL of 10mM pH6.5 phosphate buffer solution shaken in a constant temperature water bath at 50 degrees Celsius with a micro-syringe, To obtain the ginsenoside Rg3 liposome suspension, the obtained liposome suspension was homogenized by a high-pressure homogenizer for 3 cycles, and then passed through a 100nm polycarbonate membrane for 2 times under high pressure by an extruder , select the ultrafiltration membrane with molecular weight cut off 5000, remove ethanol with the mode of ultrafiltration concentration, finally gained liposome encapsulation rate is 81.2%, and average particle diameter is 105nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com