Application of salvianolate in preparation of kidney protective agent drugs
A technology of salvianolate and protective agent, which is applied in the application field of salvianolate and salvianolate magnesium in the preparation of renal protectant drugs, and can solve the problem that there are no reports to study the influence of salvianolate magnesium on renal microcirculation, etc. problem, to achieve the effect of great clinical application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
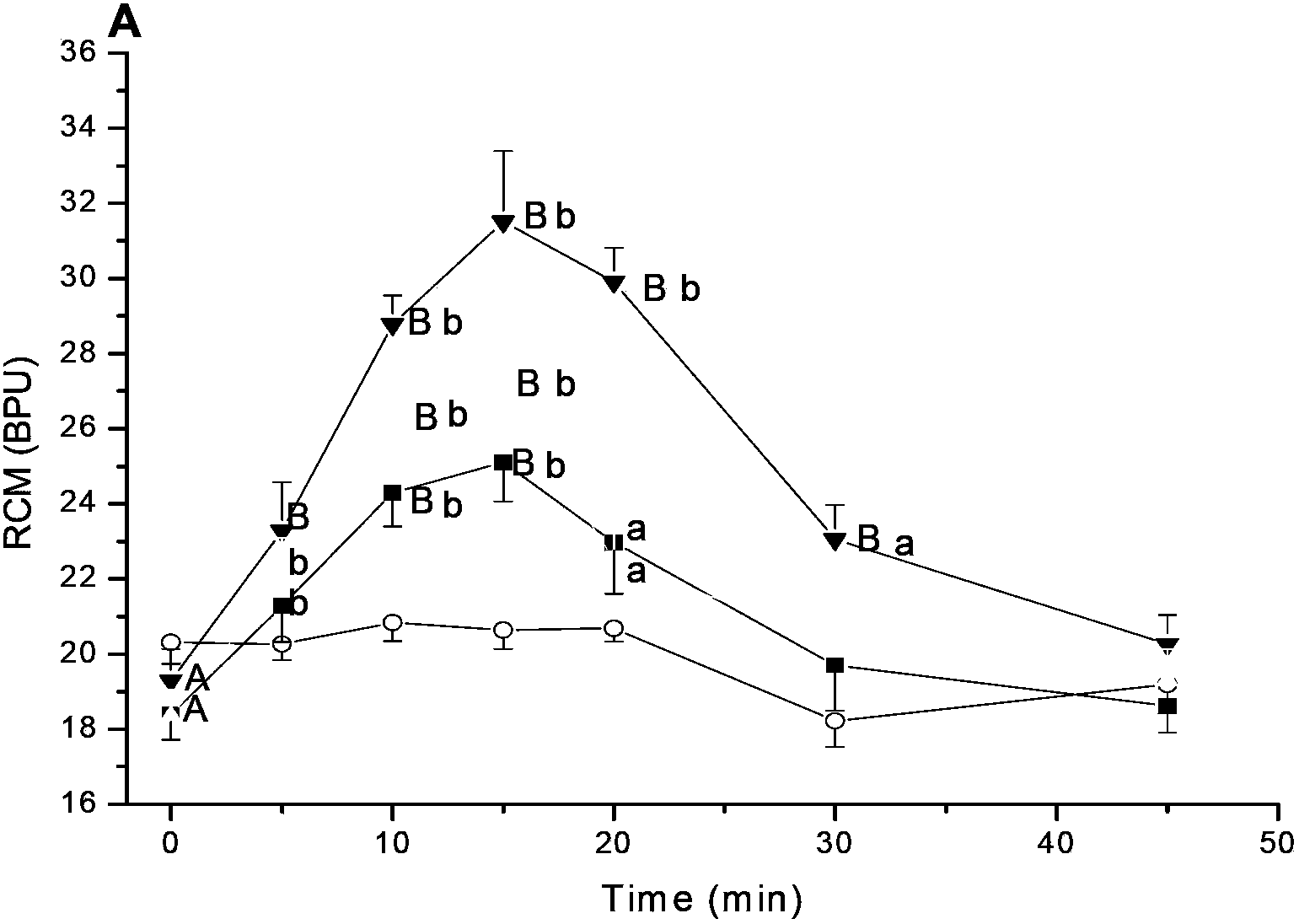
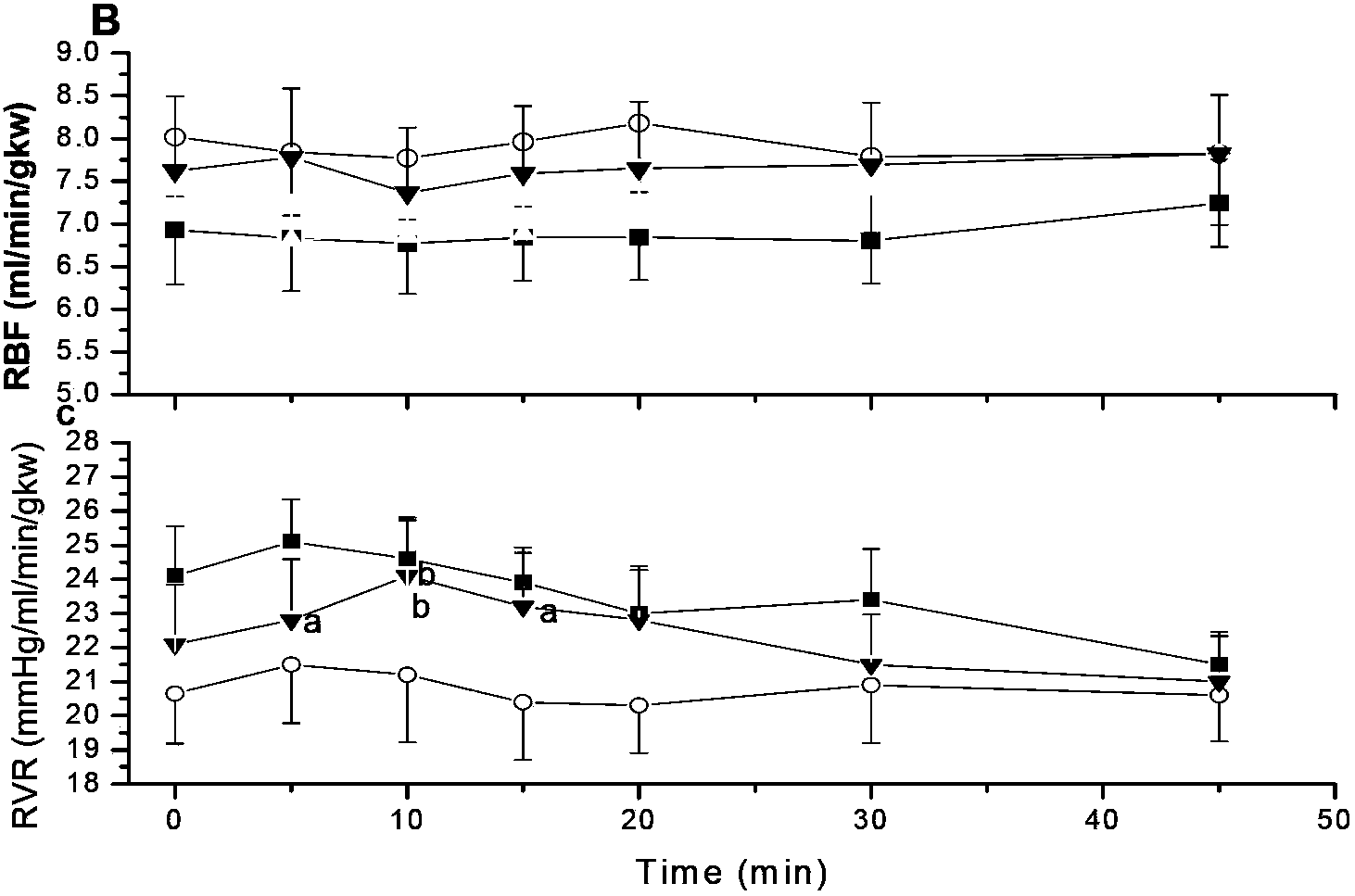
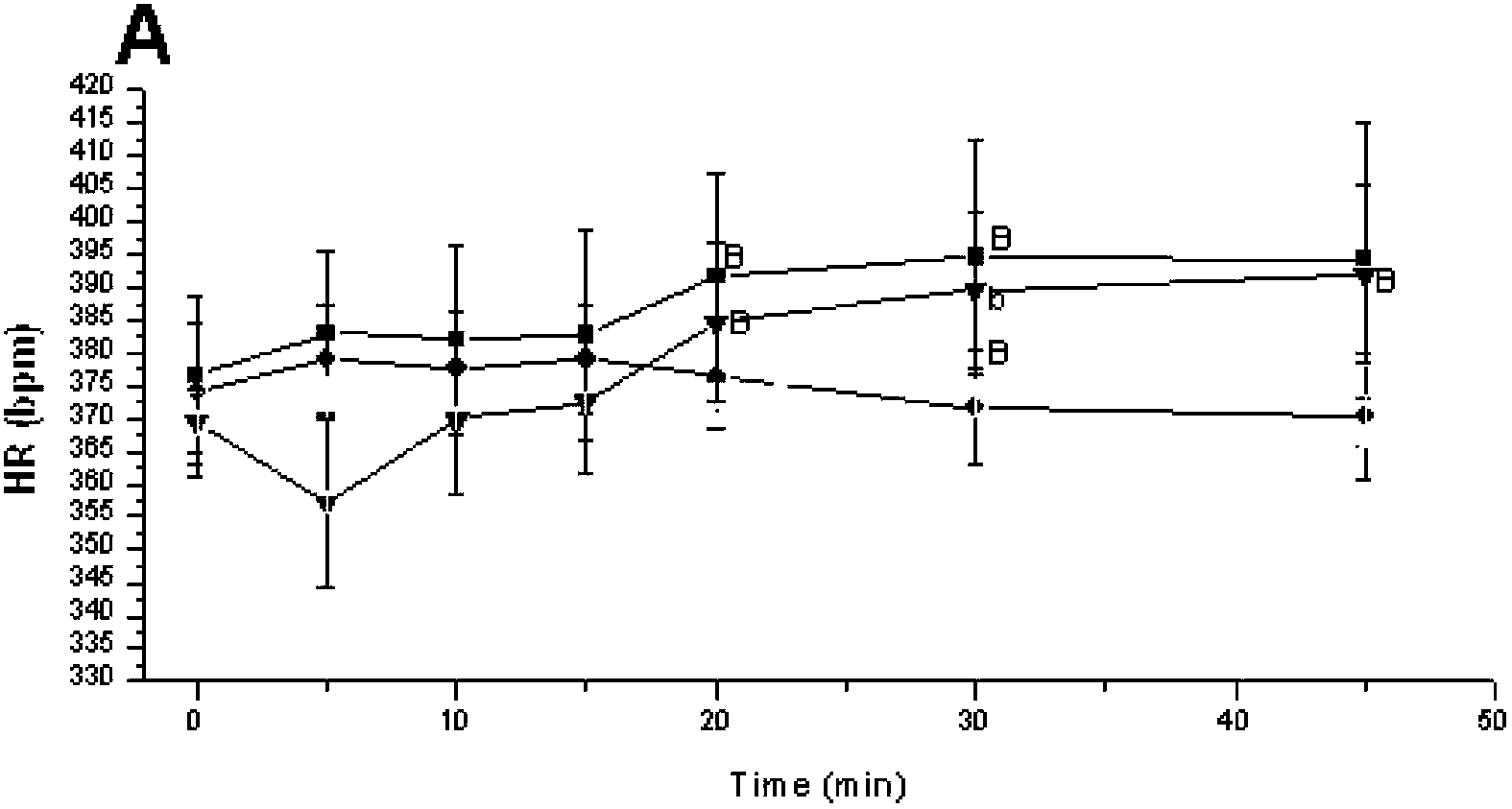
[0039] Effects of Salvia Magnesium Acetate on Microcirculation of Renal Cortex in SHR Rats
[0040] The kidneys play a decisive role in maintaining the body's water-salt balance. A highly regulated microcirculation and homeostasis are essential for the maintenance of optimal renal function. The renal microcirculation requires an intrinsic dynamic response to maintain an appropriate renal environment. This experiment was to study the effect of magnesium salvianolic acid B on renal microcirculation and hemorheology in hypertensive rats.
[0041] 1. Materials and Methods
[0042] 1.1 Reagents and medicines
[0043] Magnesiumlithospermate B (MLB; 95% purity) was produced by Shanghai Lvgu Pharmaceutical Co., Ltd. and was light brown in color. Prepare with normal saline before use. Inject 5ml of normal saline into the vial to dilute and suck it out, then inject the diluted liquid into 250ml of normal saline. All reagents are commercially available analytically pure products.
...
Embodiment 4
[0069] Preventive and therapeutic effect of salvianolate for injection on renal injury induced by contrast medium
[0070] Salvianolate for injection is produced by Shanghai Green Valley Pharmaceutical Co., Ltd., and its specifications are 50mg per bottle (containing 40mg of salvia acetic acid magnesium), 100mg per bottle (containing 80mg of salvia acetic acid magnesium), and 200mg per bottle (containing Magnesium 160mg). The implementation standard is the State Food and Drug Administration standard YBZ09012005-2010Z.
[0071] The resulting data is as follows:
[0072] Table 3 List of positive (different) serum creatinine before treatment and after treatment (7 days after operation)
[0073]
[0074] Table 4 List of abnormal values of serum creatinine 7 days after operation (μmol / L)
[0075]
[0076]
[0077] Note: "abnormal -" means that the abnormality has no clinical significance; "abnormal +" means that the abnormality has clinical significance
[0078] Tabl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com