Preparation of rod-shaped nano tungsten trioxide and technology of catalytic synthesis of adipic acid therethrough
A technology of nano-tungsten trioxide and adipic acid, which is applied in the direction of oxidative preparation of carboxylic acid, tungsten oxide/tungsten hydroxide, organic chemistry, etc., which can solve the problem of low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] 6.6 g Na 2 WO 4 . 2H 2 O (0.02 mol) was added to 50 mL of water, and HCl (9 mL) with a metering ratio of 1:1 was added dropwise under the condition of magnetic stirring. After stirring evenly, 5.48 g (0.04 mol) of p-aminobenzoic acid was added, stirred for another 0.5 h, and the mixture was poured into the reaction kettle. React at 180°C for 24 h in a blast oven. The obtained substance was repeatedly washed with ethanol and water, filtered with suction, and dried to obtain the final product.
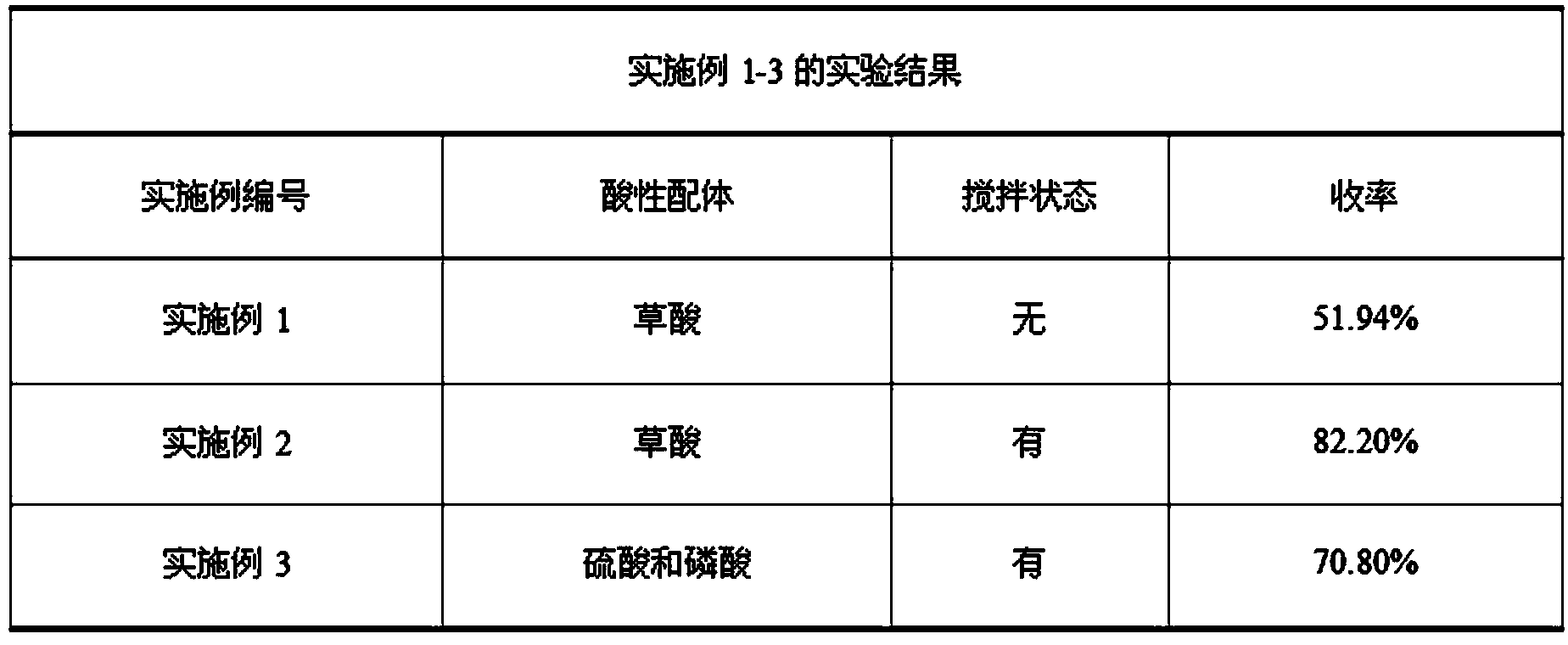
[0020] 0.0356 g of the catalyst synthesized above, 0.1 g of oxalic acid, 4.1 ml of 30% H 2 o 2 and 8 mmol cyclohexene into a 10mL reaction vessel, put the reaction kettle in an oven at 90°C for 12 h, add a certain amount of acetone after the reaction, centrifuge the mixed solution to remove the catalyst, take the supernatant, spin evaporate, and air-dry , to obtain the product. The product is weighed, and the yield is calculated, and the results are shown in the attached ...
Embodiment 2
[0022] Accurately weigh 33 g Na 2 WO 4 . 2H 2 O (0.1 mol) was added to 250 mL of water, and HCl (45 mL) with a metering ratio of 1:1 was added dropwise under the condition of magnetic stirring. After stirring evenly, 27.4 g (0.2 mol) of p-aminobenzoic acid was added, stirred for another 0.5 h, and the mixture was poured into the reaction kettle. React at 180°C for 24 h in a blast oven. The obtained substance was repeatedly washed with ethanol and water, filtered with suction, and dried to obtain the final product.
[0023] Add 1.5 mmol of the catalyst synthesized above, 0.4 mol of 30% H to the reaction vessel 2 o 2 , 0.12 mol oxalic acid, stirred at room temperature for 15 min, then added the calculated amount of cyclohexene, stirred vigorously, heated at 73°C for 2 h, then rose to 90°C and refluxed for 10 h. After the reaction, a transparent liquid was obtained by suction filtration, which was left standing at 0°C overnight, and a large amount of white crystals were pr...
Embodiment 3
[0025] Accurately weigh 33 g Na 2 WO 4 . 2H 2O (0.1 mol) was added to 250 mL of water, and HCl (45 mL) with a metering ratio of 1:1 was added dropwise under the condition of magnetic stirring. After stirring evenly, 27.4 g (0.2 mol) of p-aminobenzoic acid was added, stirred for another 0.5 h, and the mixture was poured into the reaction kettle. React at 180°C for 24 h in a blast oven. The obtained substance was repeatedly washed with ethanol and water, filtered with suction, and dried to obtain the final product.
[0026] Add 1.5 mmol of the catalyst synthesized above, 0.4 mol of 30% H to the reaction vessel 2 o 2 , 0.196 g sulfuric acid, 0.0161 g phosphoric acid, stirred at room temperature for 15 min, then added the calculated amount of cyclohexene, stirred vigorously, heated at 73 °C for 2 h, then rose to 90 °C and refluxed for 10 h. After the reaction, a transparent liquid was obtained by suction filtration, which was left standing at 0°C overnight, and a large amou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com