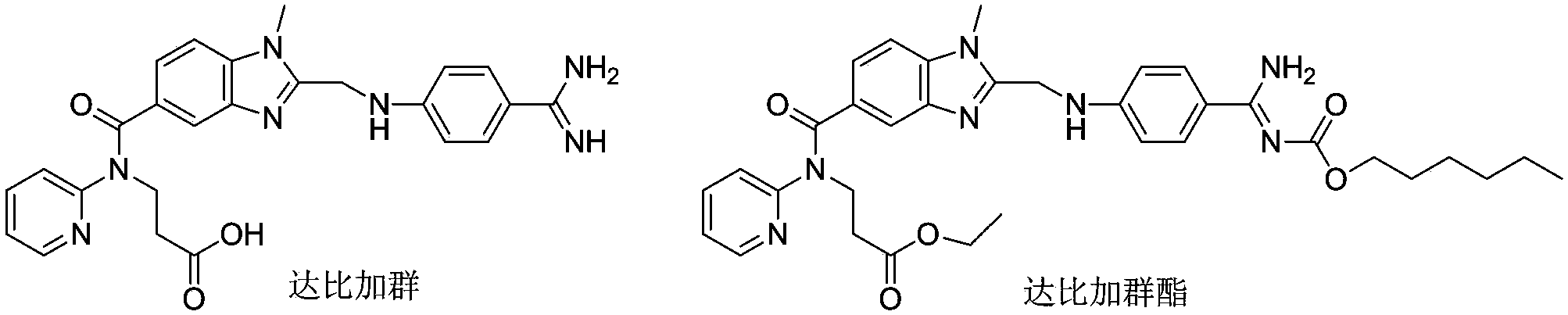
Dabigatran derivative used as prodrug, and preparation method and application thereof
A technology of dabigatran and ester derivatives, which is applied in the field of drug synthesis and can solve the problems of low oral bioavailability of dabigatran etexilate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
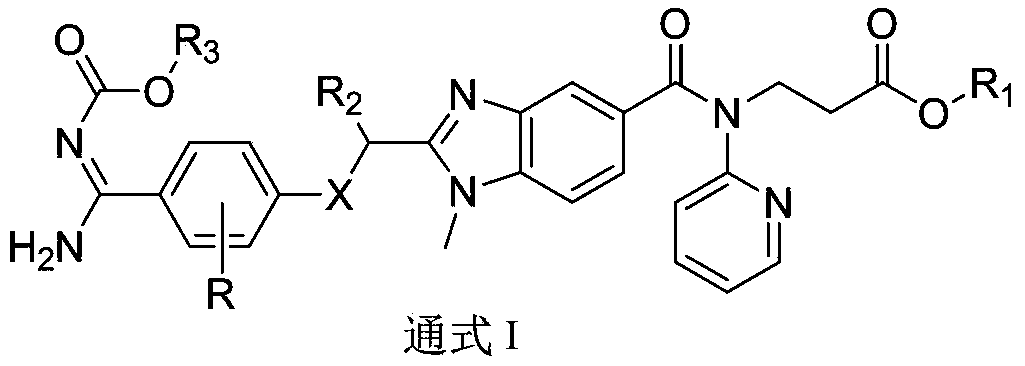
[0057] Example 1 (trans) 3-(2-((3-fluoro-4-(N'-((hexyloxy)carbonyl)formamidino)phenoxy)methyl)-1-methyl-N-( Pyridin-2-yl)-1H-benzimidazole-5-carboxamido)methyl propionate (Ⅰ 2 ) preparation
[0058] 1) 3-(2-((4-cyano-3-fluorophenoxy)methyl)-1-methyl-N-(pyridin-2-yl)-1H-benzimidazole-5-carboxamide ) Synthesis of ethyl propionate (2A)
[0059] 2-(4-cyano-3-fluorophenoxy)acetic acid (1.94g, 0.01mol), EDC1 (1.9g, 0.01mol), 1-hydroxybenzotriazole (1.35g, 0.01mol) were dissolved in THF ( 35mL) and DMF (5mL) mixture. Stir in an ice-water bath for 35 min, warm to room temperature, and slowly add 1 (3.1 g, 0.009 mol) in THF (15 mL) dropwise. Add and stir for 6h. The solvent was evaporated, dichloromethane (30 mL) was added, washed with saturated brine (5 mL x 3), dried over anhydrous sodium sulfate and filtered, the filtrate was concentrated to dryness, glacial acetic acid (45 mL) was added to the residue, and heated to reflux for 2 h. Concentrate to dryness under reduced pressur...
Embodiment 2-32
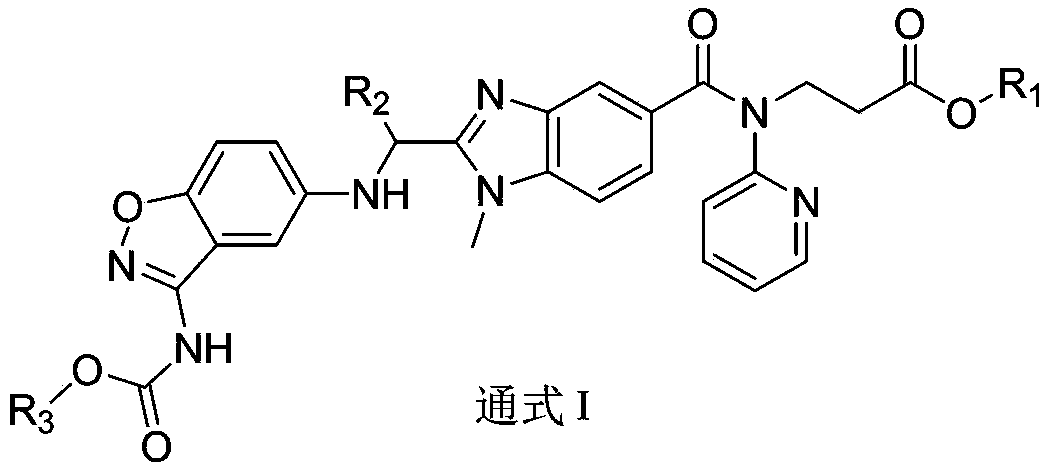
[0067] With reference to the operation of Example 1, the difference is that different phenoxyacetic acids are used, and different carboxylates react with different activated ester side chains to obtain the compound of the following formula I.
[0068]
[0069]
[0070]
[0071]
[0072]
Embodiment 33
[0073] Example 33 Evaluation of Anticoagulant Activity - Determination of Activated Partial Thromboplastin Time (aPPT)
[0074] Kunming mice with a mass of 18-20 g were divided into random groups, 10 in each group, and fasted overnight. Suspend or dissolve dabigatran etexilate and the target compound to be tested in 1% aqueous solution of sodium carboxymethyl cellulose to make a concentration of 1 mg / mL, and calculate according to a dose of 10 mg / Kg (converted to dabigatran) ) for intragastric administration, half an hour later blood was collected by cardiac puncture, 4% sodium wolfberry solution was added to a final concentration of 0.4% for anticoagulation, centrifuged at 12000r / min for 5 minutes, 0.1mL of plasma was taken, 0.1mL of aPPT reagent was added, and 37°C After pre-warming for 3 minutes, add 0.1 mL of calcium chloride solution pre-warmed at 37°C, and measure the coagulation time with a platelet aggregation coagulation factor analyzer, which is the aPPT value. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com