Wet selective desulfurization system and wet selective desulfurization method
A desulfurization system and selective technology, applied in the field of environmental chemistry, can solve non-renewable problems, achieve the effects of reducing acid consumption, high operating efficiency, and high resource utilization efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
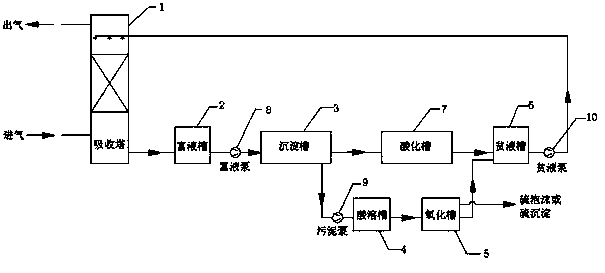
[0032] (1) Absorption of hydrogen sulfide: the raw material gas enters from the bottom of the absorption tower 1, and passes through the packing layer to make reverse contact with the desulfurization liquid filled in the packing layer. In this embodiment, the desulfurization liquid is 5g / L Ca(NO 3 ) 2 , the hydrogen sulfide in the feed gas is absorbed by the desulfurization liquid, and the purified gas after desulfurization is discharged from the top of the absorption tower 1, and the pH value of the desulfurization liquid is 5;
[0033] (2) Precipitation of metal sulfides: the desulfurized liquid that has absorbed hydrogen sulfide described in step (1) is pumped from the absorption tower 1 into the rich liquid tank 2 through the rich liquid pump 8 as the rich liquid, and enters the sedimentation tank 3 after buffering and staying, Add lye into the settling tank 3, in the present embodiment, the lye is 20g / L NaOH, under the condition of stirring, the pH value of the rich solut...
Embodiment 2
[0043] (1) Absorption of hydrogen sulfide: the raw material gas enters from the bottom of the absorption tower 1, and passes through the packing layer to make reverse contact with the desulfurization liquid filled in the packing layer. In this embodiment, the desulfurization liquid is 25g / L CaSO 4 and 18g / L CaCl 2 The hydrogen sulfide in the feed gas is absorbed by the desulfurization liquid, and the purified gas after desulfurization is discharged from the top of the absorption tower 1, and the pH value of the desulfurization liquid is 6;
[0044] (2) Precipitation of metal sulfides: the desulfurized liquid that has absorbed hydrogen sulfide described in step (1) is pumped from the absorption tower 1 into the rich liquid tank 2 through the rich liquid pump 8 as the rich liquid, and enters the sedimentation tank 3 after buffering and staying, Add alkali lye in described precipitation tank 3, in the present embodiment, described alkali lye is 35g / L Na 2 CO 3 , in the case of ...
Embodiment 3
[0054] (1) Absorption of hydrogen sulfide: The raw material gas enters from the bottom of the absorption tower 1, passes through the packing layer and contacts the desulfurization liquid filled in the packing layer in reverse. In this embodiment, the desulfurization liquid is 10g / L Ca(NO 3 ) 2 , 5g / L MnCl 2 , 20g / L Na 2 CO 3 The hydrogen sulfide in the feed gas is absorbed by the desulfurization liquid, and the purified gas after desulfurization is discharged from the top of the absorption tower 1, and the pH value of the desulfurization liquid is 7;
[0055] (2) Precipitation of metal sulfides: the desulfurized liquid that has absorbed hydrogen sulfide described in step (1) is pumped from the absorption tower 1 into the rich liquid tank 2 through the rich liquid pump 8 as the rich liquid, and enters the sedimentation tank 3 after buffering and staying, Add alkali lye in described precipitation tank 3, in the present embodiment, described alkali lye is 45g / L Na 2 CO 3 and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com