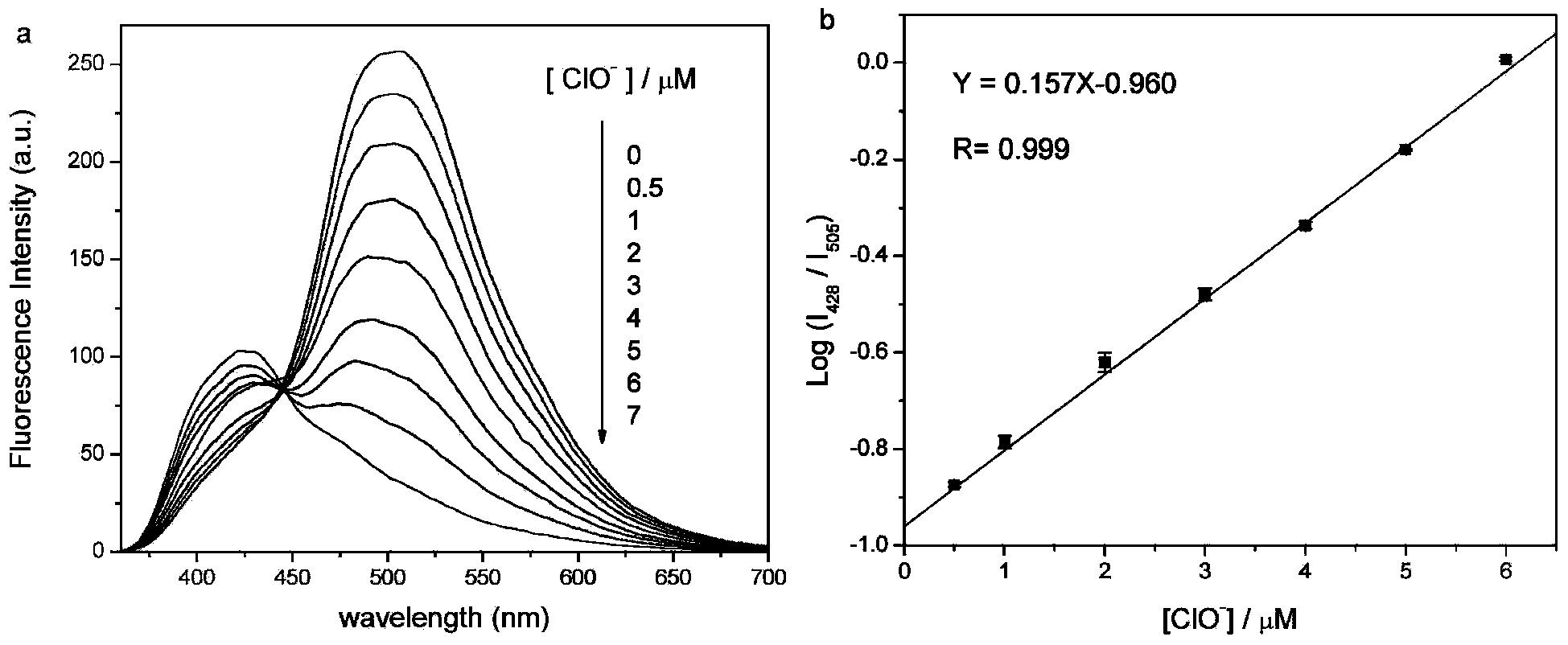
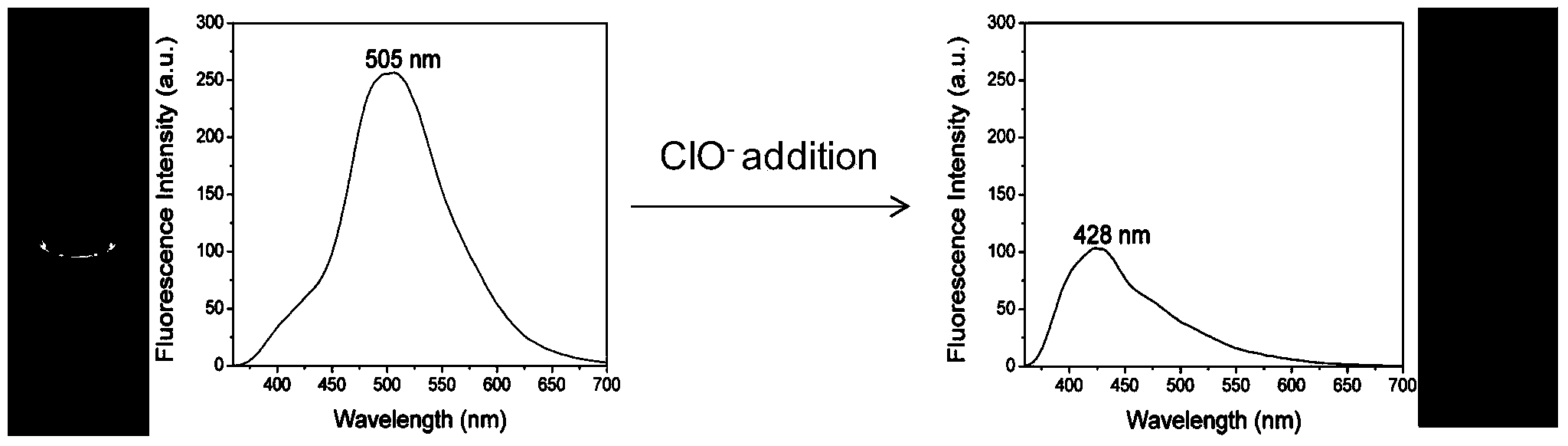
Functional multi-nitrogen heterocyclic ring fluorescent probe, preparation method and application thereof
A fluorescent probe and nitrogen heterocycle technology, which is applied in the field of functionalized multiple nitrogen heterocycle fluorescent probes, can solve problems such as damage to the body, and achieve the effects of avoiding interference, good selectivity and remarkable effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Fluorescent Probe R 1 R 2 R 3 R 4 (Py) 2 CH 2 CHNCS (R 1 , R 2 , R 3 , R 4 Both -H) Preparation
[0038]
[0039] Place a 10mL single-necked round-bottom flask in an ice-water bath, add 1mL of dichloromethane, 1mL of ethanol, and 1.0mmol of (Py) 2 (CH 2 ) 2 NH and 1.0mmol of ammonia water, the molar amount of ammonia water is expressed as NH 3 ·H 2 O meter, stirred for 30 minutes, then added CS dropwise 2 solution, the CS 2 The solution is composed of 1.1mmol of CS 2 Dissolve in 1mL of ethanol and 1mL of dichloromethane to obtain, stir and react in an ice-water bath for 1 hour after dropping, remove the ice bath, and continue to react for 9 hours at room temperature, the whole reaction process is protected from light; after the reaction is completed, the solvent is removed by rotary evaporation Obtain the crude product, the crude product is dissolved with a very small amount of dichloromethane, then separated and purified by column chromato...
Embodiment 2
[0044] Example 2: Fluorescent Probe R 1 R 2 R 3 R 4 (Py) 2 CH 2 CHNCS (R 1 =R 4 =-H,R 2 =R 3 =-OH) preparation
[0045]
[0046] Place a 10mL single-necked round-bottom flask in an ice-water bath, add 1mL of dichloromethane, 1mL of ethanol, and 1.0mmol of (Py) 2 (CH 2 ) 2 (OH) 2 NH and 1.0mmol of ammonia water, the molar amount of ammonia water is expressed as NH 3 ·H 2 O meter, stirred for 30 minutes, then added CS dropwise 2 solution, the CS 2 The solution is composed of 1.1mmol of CS 2 Dissolve in 1mL of ethanol and 1mL of dichloromethane to obtain, stir and react in an ice-water bath for 1 hour after dropping, remove the ice bath, and continue to react for 9 hours at room temperature, the whole reaction process is protected from light; after the reaction is completed, the solvent is removed by rotary evaporation Obtain the crude product, the crude product is dissolved with a very small amount of dichloromethane, then separated and purified by column chr...
Embodiment 3
[0050] Example 3: Fluorescent Probe R 1 R 2 R 3 R 4 (Py) 2 CH 2 CHNCS (R 1 =R 4 =-H,R 2 =-OH,R 3 =-COOH) preparation
[0051]
[0052] Place a 10mL single-necked round-bottom flask in an ice-water bath, add 1mL of dichloromethane, 1mL of ethanol, and 1.0mmol of (Py) 2 (CH 2 ) 2 OHNHCOOH and 1.0mmol of ammonia water, the molar amount of ammonia water is expressed as NH 3 ·H 2 O meter, stirred for 30 minutes, then added CS dropwise 2 solution, the CS 2 The solution is composed of 1.1mmol of CS 2 Dissolve in 1mL of ethanol and 1mL of dichloromethane to obtain, stir and react in an ice-water bath for 1 hour after dropping, remove the ice bath, and continue to react for 9 hours at room temperature, the whole reaction process is protected from light; after the reaction is completed, the solvent is removed by rotary evaporation Obtain the crude product, the crude product is dissolved with a very small amount of dichloromethane, then separated and purified by column...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com