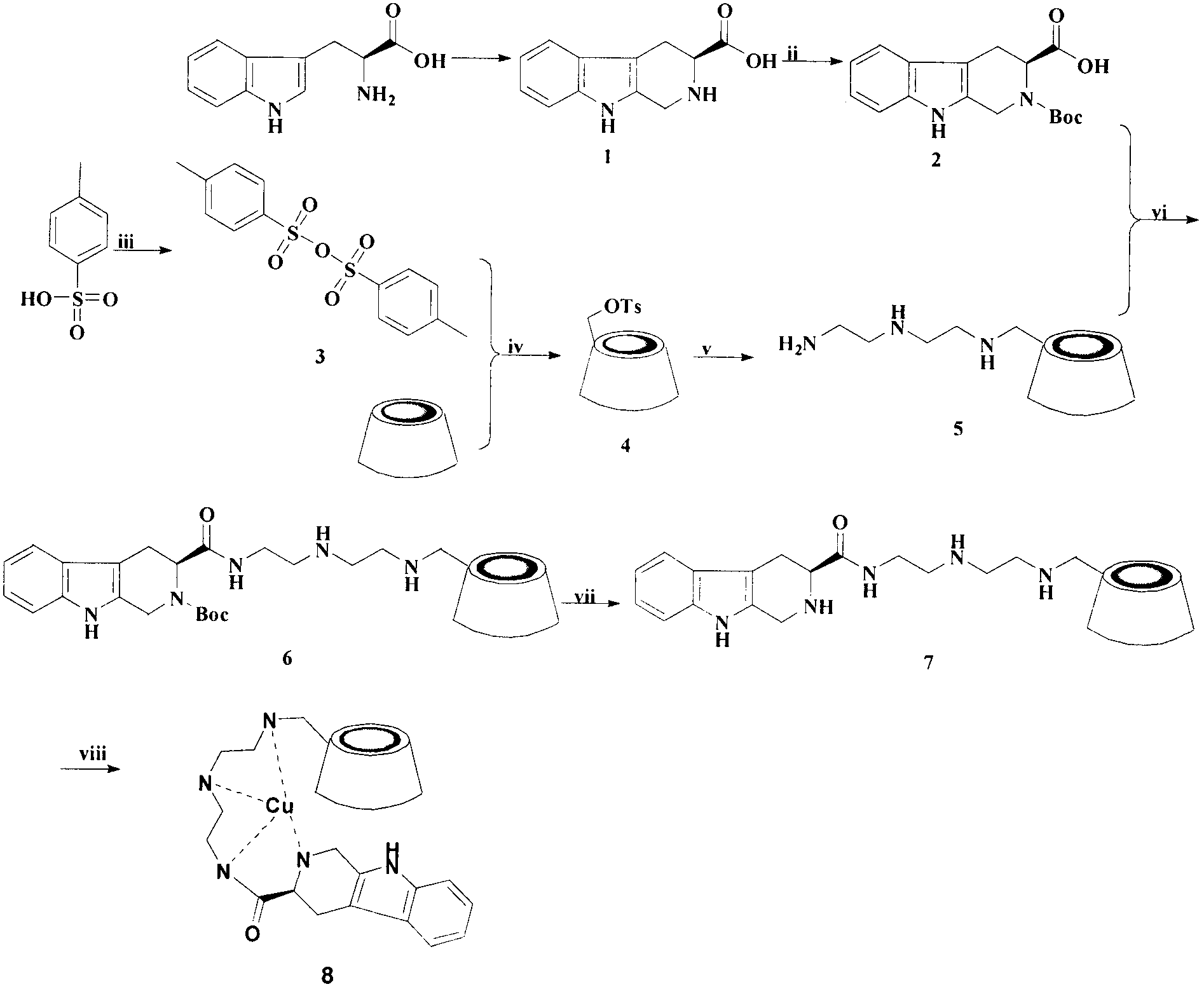
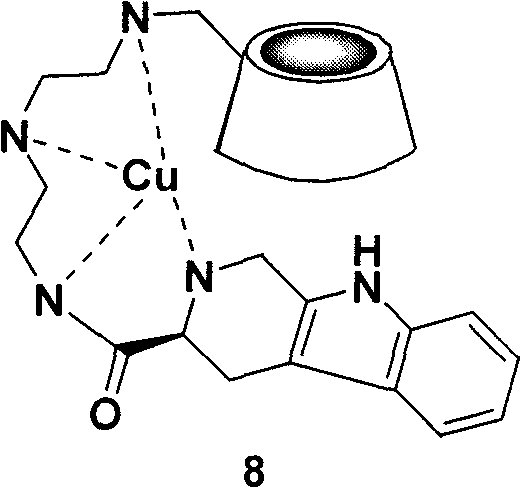
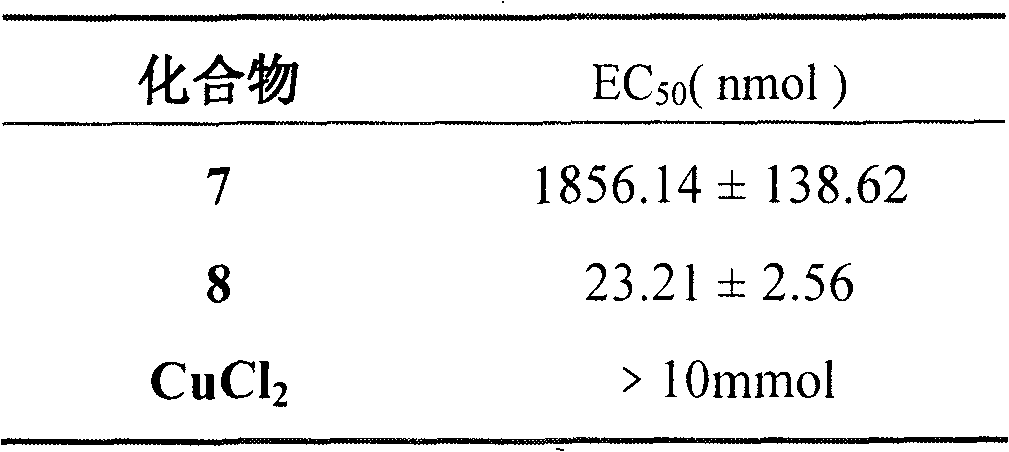
Tetrahydro-beta-carbolinyl-3- formyl-diethylenetriamino-beta-cyclodextrin copper complex, and preparation, antithrombotic action and application thereof
A technology of diethylenetriamine and cyclodextrin, which is applied in the preparation of antithrombotic drugs, in the field of modification and complexation of β-cyclodextrin, can solve the problems of limited application of water solubility, and achieve improved antithrombotic The effect of thrombus capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1 prepares 1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid (1)
[0023] Add 0.1 mL of concentrated sulfuric acid dropwise to 200.0 mL of water, add 5.000 g of tryptophan (24.5 mmol), and after the tryptophan is completely dissolved, add dropwise 5.0 mL of formaldehyde. After 6 hours of reaction, the reaction was terminated, and the pH value of the system was adjusted to 6 with concentrated ammonia water, resulting in a large amount of white precipitate. The reaction mixture was frozen in a refrigerator at 4°C for several hours, and filtered to obtain 3.985 g of the title compound, with a yield of 75%. ESI / MS(m / e): 217[M+H] + .
Embodiment 2
[0024] Embodiment 2 prepares N-Boc-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid (2)
[0025] 1,2,3,4-Tetrahydro-β-carboline-3-carboxylic acid (5.000g, 23.1mmol) was suspended in 50.0mL DMF, and Boc was added under ice-cooling 2 O (6.545g, 30.1mmol), triethylamine was added dropwise to adjust the pH of the reaction system to 10. After the reaction is complete, dry DMF, dissolve the product mixture in ethyl acetate, wash three times with 5% potassium bisulfate, and wash twice with saturated NaCl solution until the pH of the ester layer is neutral, and anhydrous Na 2 SO 4 Dry the ester layer. After the desiccant was filtered off, the ethyl acetate was spin-dried, and the pale yellow solid was washed with a small amount of chloroform until it became colorless to obtain 4.308 g of the title compound, with a yield of 59%. ESI / MS(m / e): 317[M+H] + .
Embodiment 3
[0026] Embodiment 3 prepares 4-toluenesulfonic anhydride (3)
[0027] TsCl (8.000g, 42.0mmol) and TsOH·H 2 O (2.000 g, 11 mmol) was dissolved in 50.0 mL of dichloromethane and stirred overnight at room temperature. After the reaction solution was filtered and recrystallized, 4.970 g of the title compound was obtained, with a yield of 62%. ESI / MS(m / e): 327[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com