Synthetic method of ziprasidone intermediate
A kind of technology of ziprasidone and synthesis method, applied in the field of synthesis of ziprasidone intermediate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
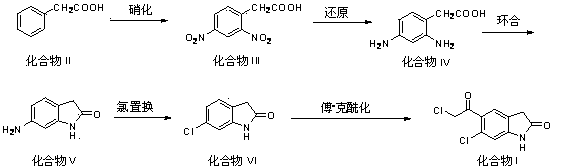
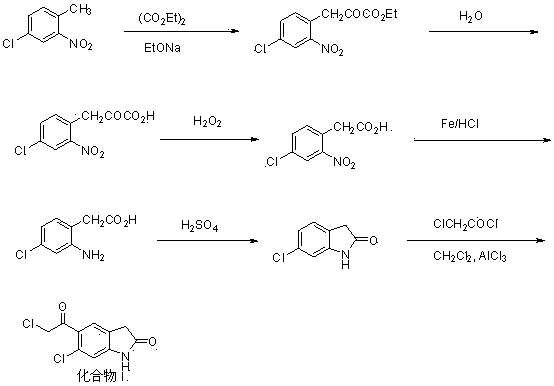
[0035] Embodiment 1: Preparation of compound III by nitration of compound II
[0036] Example 1-1 Add 16 g of concentrated nitric acid and 32 g of concentrated sulfuric acid into the reaction flask. After the temperature drops to room temperature, add 35 g of phenylacetic acid (compound II, 257 mmol) in portions under stirring. The reaction is exothermic violently. The temperature of the reaction was 80°C and the temperature was maintained for 1 hour. The reaction solution was poured into 100 g of ice water with stirring, and a yellow solid was precipitated, which was filtered, washed with water and dried. Recrystallization from ethanol gave 51.8 g of compound III yellow needle crystals, with a yield of 89.1%.
[0037] Example 1-2 Add 64 g of concentrated nitric acid and 32 g of concentrated sulfuric acid into the reaction flask. After the temperature drops to room temperature, add 35 g of phenylacetic acid (compound II, 257 mmol) in portions under stirring. The reaction is e...
Embodiment 2
[0040] Embodiment 2: Compound III is reduced to prepare compound IV
[0041]Example 2-1 Add 150 mL of water to the reaction flask, heat to 40°C, add 17.0 g (143 mmol) of tin powder and adjust the pH to 3 with concentrated hydrochloric acid, continue to heat up to 60°C, add 13.0 g (57.4 mmol) and 23.9 g (201 mmol) of tin powder, after adding, react at 60~80°C for 8 hours, then add 10% sodium hydroxide solution, adjust the pH to 8~9, and stir for 30 minutes. After suction filtration, the filtrate was acidified with concentrated hydrochloric acid to pH 4~5, and a yellow solid precipitated out. The solid was collected and vacuum-dried to obtain 8.7 g of compound IV yellow solid, with a yield of 90.6%.
[0042] Example 2-2 Add 150 mL of water to the reaction flask, heat to 40°C, add 9.3 g (143 mmol) of zinc powder and 150 mL of acetic acid, continue to heat up to 60°C, add 13.0 g (57.4 mmol) of compound III in batches ) and 13.1 g (201 mmol) of iron powder, after adding, react at ...
Embodiment 3
[0048] Embodiment 3: compound IV cyclization preparation compound V
[0049] Example 3-1 Add 20 g (120 mmol) of compound IV into 30 mL of toluene, stir to dissolve completely, raise the temperature to 110°C, then add 7.0 mL (12.9 g, 132 mmol) of concentrated sulfuric acid dropwise, keep the reaction for 2 hours, pump Filter, wash the solid with water, and dry in vacuo to obtain 15.5 g of compound V as a yellow solid, with a yield of 86.7%.
[0050] Example 3-2 Add 20 g (120 mmol) of compound IV into 30 mL of toluene, stir to dissolve completely, raise the temperature to 110°C, then add 16 mL (29.4 g, 300 mmol) of concentrated sulfuric acid dropwise, keep the reaction for 2 hours, pump Filtered, washed the solid with water, and dried in vacuo to obtain 16.3 g of compound V yellow solid, with a yield of 91.5%.
[0051] Example 3-3 Add 20 g (120 mmol) of compound IV into 30 mL of toluene, stir to dissolve completely, raise the temperature to 110°C, then add 15.7 mL (18.5 g, 188 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com