Low polymerization volume shrinkage (meth)acrylate monomer without bisphenol A structure, preparation method and application thereof
A technology of volume shrinkage and acrylate, applied in dental preparations, dental prostheses, pharmaceutical formulations, etc., can solve problems such as low polymerization volume shrinkage, achieve high double bond conversion rate, small polymerization volume shrinkage, and mechanical properties Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 ACD monomer
[0037] The preparation method of the present embodiment ACD monomer comprises the steps:
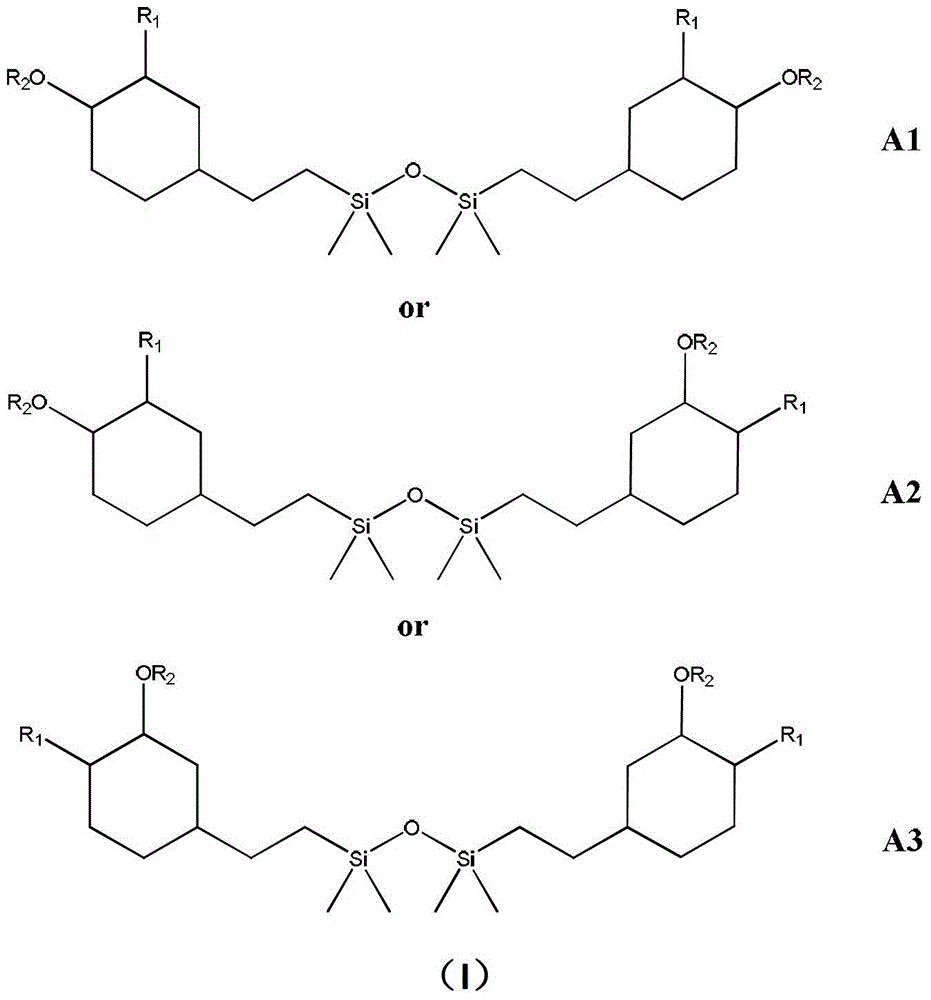
[0038] Step 1: Add 9.276g1,3-bis[2-(3,4-epoxycyclohex-1-yl)ethyl]tetramethyldisiloxane and 4.275g Methacrylic acid, add 100ml of dichloromethane, 0.050g N,N-dimethylbenzylamine, 0.06g hydroquinone, reflux and stir at 90°C for 24 hours, cool to room temperature, and then carry out acid solution on the reaction product Washing lye washing purification treatment.
[0039] The product was characterized by infrared and NMR:
[0040] 1 H-NMR (400MHz, CCl 3 D): δ6.14(s, 2H), δ5.60(s, 2H), δ3.95-3.15(m, 4H), δ2.01(s, 6H), δ1.90-1.45(m, 14H) ), δ1.45-1.20 (m, 8H), 0.50 (t, 12H).
[0041] FT-IR: ν(cm -1 ) 3648-3134, 2920, 2854, 1720, 1637, 1253, 1173, 795, 650.
Embodiment 2
[0042] Example 2 Polymerization volume shrinkage of dental resin containing ACD monomer
[0043] In this example, the ACD monomer was mixed with the diluent TEGDMA commonly used in dental restoration materials, and the resin system Bis-GMA / TEGDMA commonly used in dental restoration materials was used as a control group to study the polymerization volume shrinkage of the prepared resin after curing.
[0044] The resin formula and the results of polymerization volume shrinkage are shown in Table 1 to Table 2.
[0045] Table 1 Resin composition containing ACD monomer
[0046]
[0047] Table 2 Polymerization volume shrinkage of resin system containing ACD monomer and reference resin system
[0048]
[0049] It can be seen that, compared with Bis-GMA, the resin system with ACD monomer content of 51.2% has the lowest polymerization shrinkage.
[0050] The polymerization shrinkage is mainly caused by the change of the distance between molecules before and after the polymeriza...
Embodiment 3
[0051] Embodiment 3 Contains the mechanical properties of ACD monomer dental resin
[0052] In this example, on the basis of Example 3, the cured mechanical properties of the resin system prepared in Example 3 were studied.
[0053] The mechanical performance test results are shown in Table 3.
[0054] Table 3 Mechanical properties of the resin system containing ACD monomer and the reference resin system
[0055]
[0056] It can be seen that, compared with Bis-GMA, the mechanical properties of the resin system containing ACD monomer have decreased, but it still meets the application standards of dental restoration resins.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com