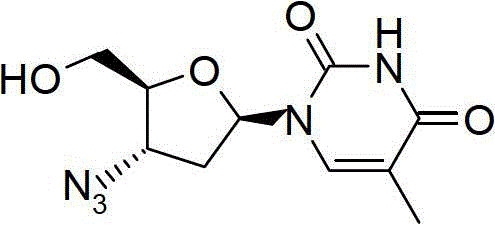
Process for the preparation of β-thymidine
A thymidine and buffer system technology, applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of low yield of β-thymidine, easy breakage of glycosidic bonds, poor purity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
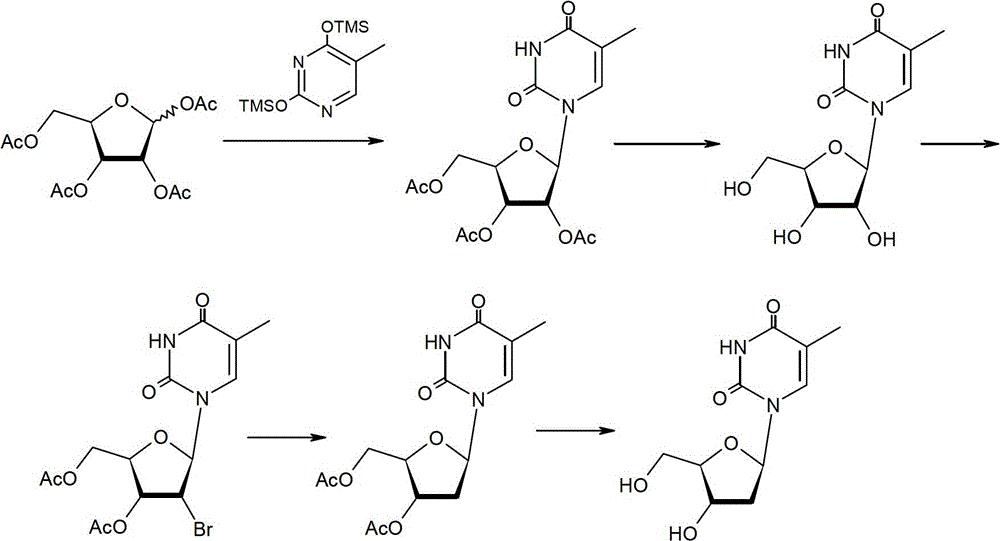
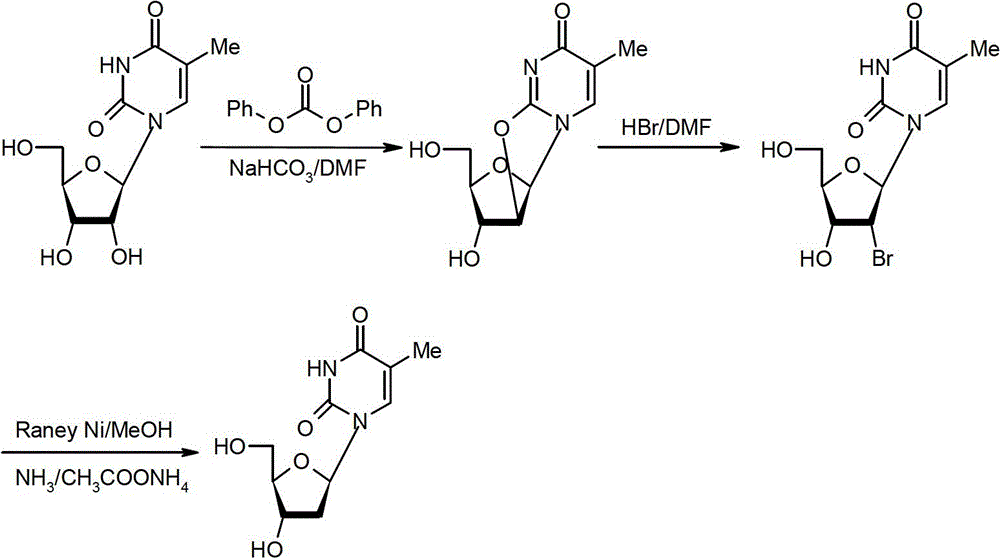
[0065] Preparation method of β-thymidine
[0066] The preparation method of β-thymidine according to the present invention comprises the steps of: in a hydrogen atmosphere, in the presence of a catalyst, at a certain temperature (20-60°C, preferably 20-40°C), at a pH of 6.5- In the buffer system of 8.0, the compound represented by formula II is dehalogenated for a period of time (such as 2-20 hours, preferably 5-10 hours), so as to obtain β-thymidine represented by formula I;
[0067]
[0068] Wherein, X is Cl or Br.
[0069] In another preferred example, the dehalogenation reaction is carried out under normal pressure, that is to say, the pressure of the hydrogen atmosphere is the gas pressure produced by the atmosphere we usually live in, which is about a standard atmospheric pressure (101.325KPa) .
[0070] In another preferred example, the pH of the buffer system is 7.0-8; more preferably 7.0-7.6.
[0071] In another preferred embodiment, when the dehalogenation reac...
Embodiment 1
[0097] Preparation of 2,2'-anhydrothymidine:
[0098] Add DMF 100mL, 5-methyluridine (50.0g, 0.19mol), sodium hydroxide (0.18g, 4.4mmol) and diethyl carbonate (46mL, 0.38mol) into the reaction flask, heat up to 120°C, keep warm The reaction was stirred for 8 hours. It was detected by TLC that the reaction of the raw materials was complete, and the temperature was lowered to room temperature, and the pH value was adjusted to neutrality (about 7.0) with 30% ethanol hydrochloric acid solution, and concentrated to dryness. Add 50 mL of ethyl acetate to the concentrate and reflux for beating for 1 hour, cool down to room temperature, filter, collect the solid, and dry the solid under vacuum at 60°C to obtain the title compound (white solid, 44.5 g), with a molar yield of 95.6% and an HPLC purity of 98.2%. MS (ESI) m / z: (M+H) 241.2, (M+Na) 263.2.
Embodiment 2
[0100] Preparation of 2'-bromothymidine:
[0101] Add 16% hydrogen bromide DMF solution (119.3g, 0.24mol) into the reaction flask, add 2,2'-dehydrothymidine (44.5g, 0.18mol) under stirring, and heat up to 40°C for 2 hours. Add 16% hydrogen bromide DMF solution (27.5 g, 0.054 mol) and continue to keep warm for 2 hours. TLC detected that the reaction of the raw materials was complete, and the DMF was distilled off under reduced pressure to obtain a viscous oily bromide, which was directly put into the next reaction. MS (ESI) m / z: (M+H) 322.2, (M+Na) 344.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com