Preparation method for adsorbent capable of removing heavy metals
A technology for adsorbents and heavy metals, applied in the field of preparation of adsorbents for removing heavy metals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0013] Embodiment 1: Preparation of manganese oxides under different pH conditions
[0014] The 1L medium for the experiment consists of: 0.5g of yeast extract, 0.5g of hydrolase protein, CaCl 2 0.222g, MgSO 4 0.796g, glucose 0.5g, FeCl 3 1ml (3.7mM), trace elements 1ml (CuSO 4 ·5H 2 O 10mM, ZnSO 4 ·7H 2 O 44mM, CoCl 2 ·6H 2 O 20mM, NaMoO 4 2H 2 O 13mM). After the preparation of the medium, adjust the pH to 6-8 with 4M NaOH, sterilize with damp heat at 115°C for 30 minutes, inoculate 2% (V / V) manganese-oxidizing bacteria Pseudomonas sp. The buffer solution of bacteria, the final concentration is 20mM (the buffer solution is 4-hydroxyethylpiperazineethanesulfonic acid (HEPES) or 2-(N-morpholine) ethanesulfonic acid (MES), wherein HEPES adjusts the pH to 6.0- 7.5, MES to adjust the pH to 8.0), 27 ° C constant temperature shaking culture for 19 hours before adding filter-sterilized MnCl 2 (The final concentration is 1mM), and continuously cultivated at 27°C for 2 w...
specific Embodiment approach 2
[0016] Specific embodiment two: Scanning electron microscope observation (SEM) and energy spectrum analysis (EDX) of manganese oxide
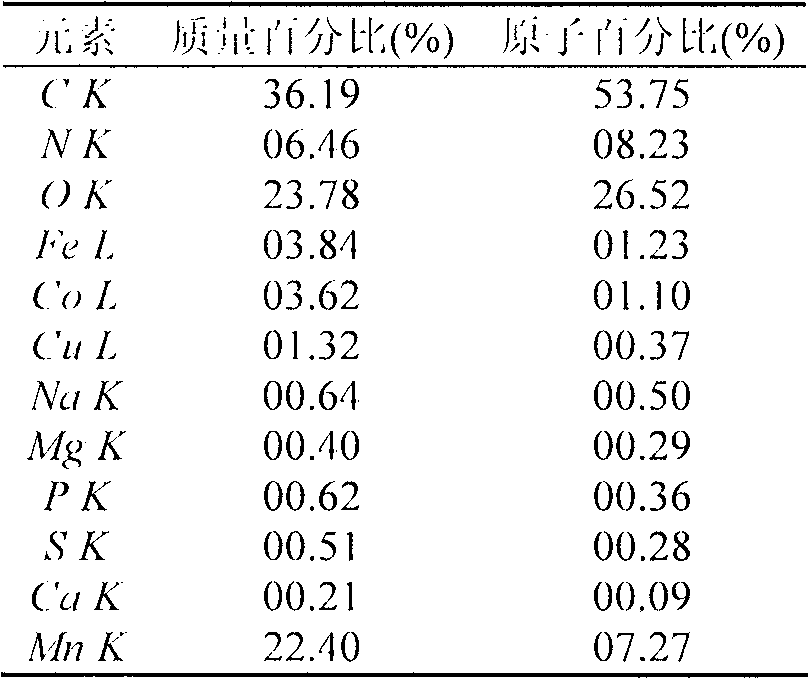
[0017] Table 1 Energy spectrum analysis results
[0018]
[0019] Note: K and L refer to the excitation energy of the K and L layers respectively.
[0020] The manganese oxide synthesized under the condition of pH 7.5 was observed by scanning electron microscope and analyzed by energy spectrum (S-3000N, HITACIII Co., Japan). The results of scanning electron microscopy and energy dispersive spectroscopy were as follows: figure 1 As shown in Table 1, aggregates of manganese oxide can be formed outside the microorganism, and a higher content of manganese oxide can be obtained through biological methods, with a mass percentage of 22.40%.
specific Embodiment approach 3
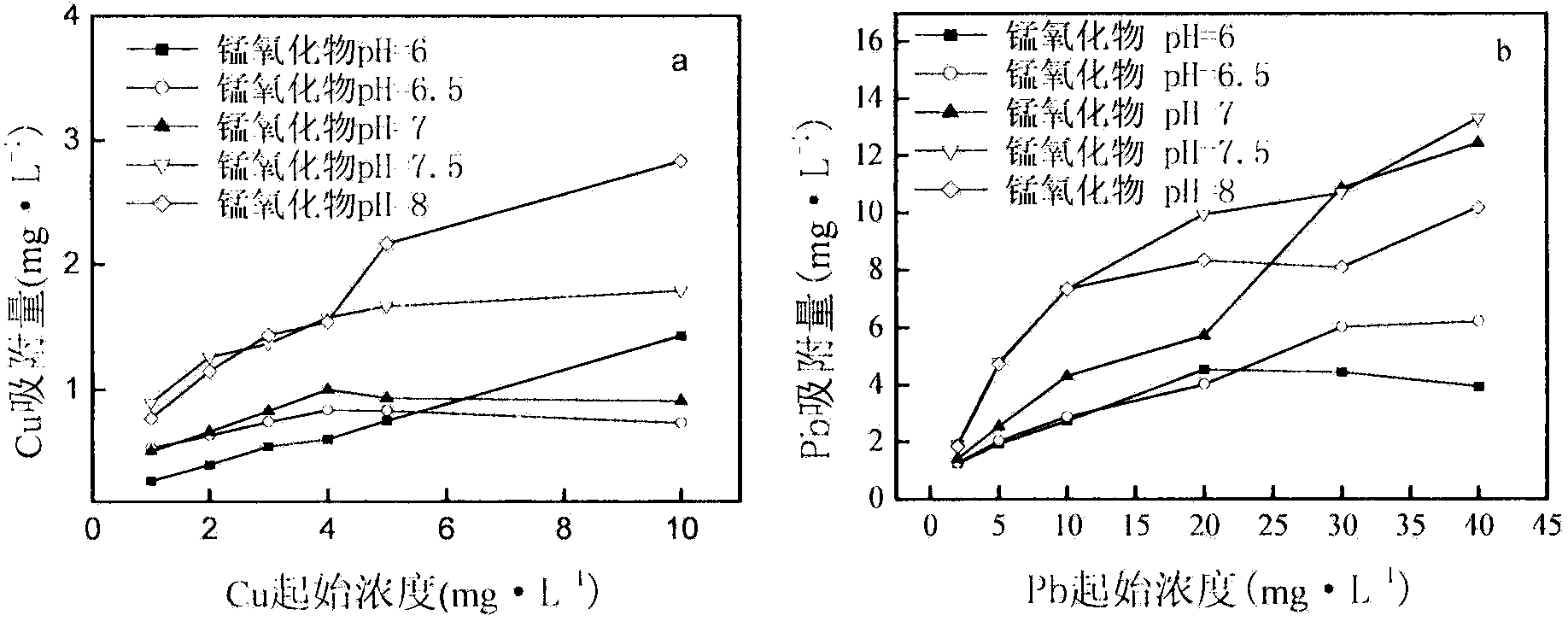
[0021] Specific embodiment three: the adsorption of manganese oxide to heavy metal Cu, Pb
[0022] Take 5ml manganese oxide suspension, add 2.5ml 0.1M NaO 3and different amounts of heavy metals, the pH was adjusted to 5.0+0.1, and the total volume was 25ml. Shake at 25°C for 24 hours, centrifuge at 13000g, and measure the concentration of heavy metal ions in the supernatant by inductively coupled plasma emission spectrometry (ICP-OES, OPTMA2000, Perk Elmer Co, U.S.A.), the difference between the concentration of heavy metals in the supernatant and the initial concentration All adsorption experiments were repeated 3 times for the adsorption amount of heavy metals on manganese oxides. It is known from the specific embodiment one that the concentration difference of manganese oxide in the suspension prepared under the conditions of pH 6.0 and 8.0 is the largest, which is 14.35%, but from figure 2 a and figure 2 It is known from b that after the adsorption reaches equilibrium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com