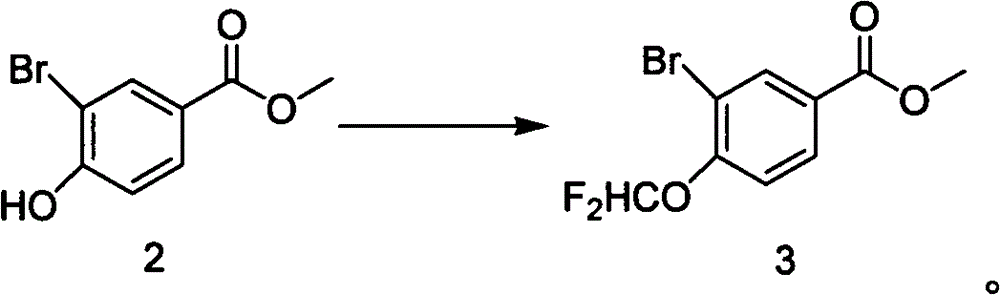
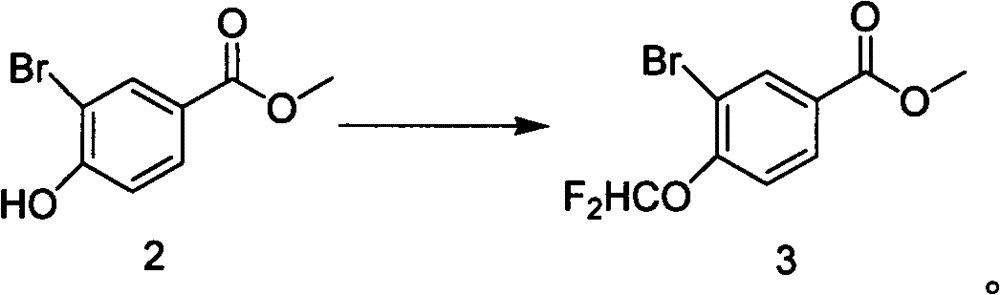
A kind of preparation method of methyl 3-bromo-4-difluoromethoxybenzoate
A technology of methyl difluoromethoxybenzoate and sodium difluorochloroacetate, which is applied in the field of preparation of methyl 3-bromo-4-difluoromethoxybenzoate, and can solve the problem of long reaction time and cumbersome post-treatment , low yield and other problems, to achieve the effect of high conversion rate, simple post-processing and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Add methyl 3-bromo-4-hydroxybenzoate (6.61g, 28.6mmol, 1eq) and sodium chlorodifluoroacetate (8.721, 57.2mmol, 2eq) into anhydrous DMF (43ml), stir until completely dissolved, Add K to it 2 CO 3 (5.933g, 42.9mmol, 1.5eq), stirred, heated to 95°C, controlled at 93-98°C, and reacted for 1.5h. After the reaction was completed, the temperature dropped below 20°C, 86ml of water was added, stirred for 1h, the solid precipitated, filtered, washed with water (2×20ml), and dried in vacuo to obtain 7.2g of white crystals, yield: 89.6%, HPLC: 99.1%, MS (EI + ): isotope peak 280:282=1:1, 1 H-NMR (CDCl 3 ): δ8.31(dd, H), 8.22(dd, 1H), 7.05(m, 1H), 6.61(t, 1H), 3.93(s, 3H).
Embodiment 2
[0019] Add methyl 3-bromo-4-hydroxybenzoate (1eq) and sodium chlorodifluoroacetate (1.5eq) into anhydrous DMF, stir until completely dissolved, and add K 2 CO 3 (1.5eq), stirred, heated to 95°C, controlled at 93-98°C, and reacted for 1-1.5h. After the reaction was completed, the temperature dropped below 20°C, 86ml of water was added, and the mixture was stirred for 1 hour. The solid was precipitated, filtered, washed with water, and dried in vacuo to obtain white crystals with a yield of 74.5%. HPLC: 92.6%, MS (EI + ): isotope peak 280:282=1:1, 1 H-NMR (CDCl 3 ): 58.31(dd,H), 8.22(dd,1H), 7.05(m,1H), 6.61(t,1H), 3.93(s,3H).
Embodiment 3
[0021] Add methyl 3-bromo-4-hydroxybenzoate (1eq) and sodium chlorodifluoroacetate (2.5eq) into anhydrous DMF, stir until completely dissolved, and add K 2 CO 3 (1.5eq), stirred, heated to 95°C, controlled at 93-98°C, and reacted for 1-1.5h. After the reaction was completed, the temperature dropped below 20°C, 86ml of water was added, stirred for 1 hour, solid precipitated, filtered, washed with water, and dried in vacuo to obtain white crystals, yield: 88.3%, HPLC: 98.7%. MS (EI + ): isotope peak 280:282=1:1, 1 H-NMR (CDCl 3 ): δ8.31(dd, H), 8.22(dd, 1H), 7.05(m, 1H), 6.61(t, 1H), 3.93(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com