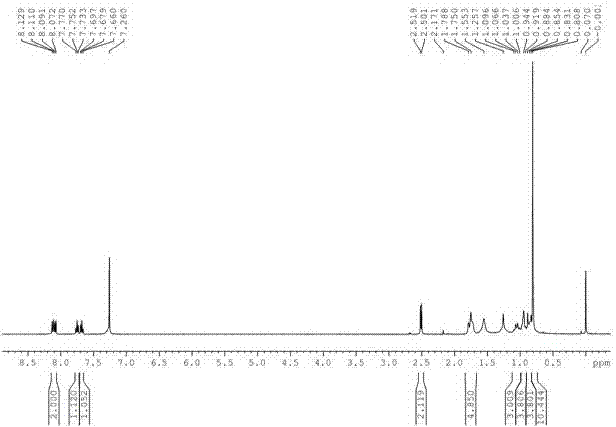
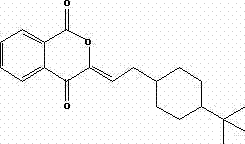
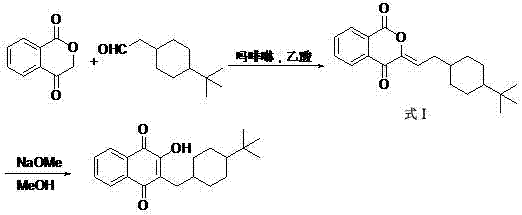
Preparation method of buparvaquone
A technology of bupavaquinone and solvent is applied in the field of drug synthesis, which can solve the problems of low yield and the like, and achieve the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Step 1: Condensation Reaction
[0028] Under argon protection, add 1.62g (0.01mol) of 1,4-benzopyrandione to a 50ml three-necked flask, slowly add 20ml of acetic acid solution of 1.82g (0.01mol) of p-tert-butylcyclohexylaldehyde, add 0.87ml (0.01mol) of isobutylamine was heated to 60°C for 4h.
[0029] Post-processing: Add 20ml of water dropwise to the reaction flask, solids are precipitated, filtered by suction, and recrystallized with 50ml of methyl tert-butyl ether to finally obtain a yellow solid product (formula intermediates shown).
[0030] Step 2: rearrangement reaction
[0031] Add the above product, 10ml of methanol, 10ml of 30% methanol solution of sodium methoxide (made now) into a 100ml single-necked bottle, and control the temperature at 20°C until the reaction is complete.
[0032] Post-processing: Filtration, adding 40ml of acetic acid to the filtrate to adjust the pH to 7-8, concentrating in vacuo until the solid precipitates, filtering with suction...
Embodiment 2
[0037] Step 1: Condensation Reaction
[0038] Under nitrogen protection, add 3.24g (0.02mol) of 1,4-benzopyrandione into a 100ml three-necked flask, slowly add 40ml of acetic acid solution of 7.28g (0.04mol) of p-tert-butylcyclohexylaldehyde, and add iso Butylamine 1.74ml (0.02mol), heated to 30°C for 10h.
[0039] Post-processing: drop 40ml of water into the reaction flask, solids are precipitated, filtered by suction, recrystallized with 100ml of ether, and finally obtain a yellow solid product (formula intermediates shown).
[0040] Step 2: rearrangement reaction
[0041] Add the above product, 20ml of methanol, and 20ml of 30% methanol solution of sodium methoxide (made now) into a 100ml single-necked bottle, and the reaction is completed at 20°C.
[0042] Post-processing: Filtration, adding 100ml acetic acid to the filtrate to adjust the pH to 7-8, concentrating in vacuo until the solid precipitates, filtering with suction, recrystallizing with 200ml of a mixed soluti...
Embodiment 3
[0044] Step 1: Condensation Reaction
[0045] Under argon protection, add 1.62g (0.01mol) of 1,4-benzopyrandione to a 50ml three-necked flask, slowly add 20ml of acetic acid solution of 1.82g (0.01mol) of p-tert-butylcyclohexylaldehyde, and add Morpholine 2.61ml (0.03mol), warmed up to 40°C for 4h.
[0046] Post-processing: Add 20ml of water dropwise to the reaction flask, solids are precipitated, filtered by suction, and recrystallized with 50ml of methyl tert-butyl ether to finally obtain a yellow solid product (formula intermediates shown).
[0047] Step 2: rearrangement reaction
[0048] Add the above product, 10ml of methanol, 10ml of 30% sodium ethoxide in ethanol (prepared now) into a 100ml single-necked bottle, and the reaction is completed at 20°C.
[0049] Post-treatment: filter, add 40ml of acetic acid to the filtrate to adjust the pH to 7~8, concentrate in vacuo until the solid precipitates, filter with suction, recrystallize with 100ml of methanol and water mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com